Coordinated scission of N-H bonds
By Trevor Brown on November 17, 2016
A paper published in this week’s edition of Science outlines a new approach to breaking the hydrogen-nitrogen bonds in ammonia, allowing the production of hydrogen at low temperatures.
This research was also reported on phys.org under the headline: “Method found for pulling hydrogen from ammonia for use as clean fuel.”
In the same issue of Science, an accompanying Perspectives article explains the implications of this research, with potential new applications for ammonia as a chemical feedstock in the pharmaceutical and materials industries, and as an “energy carrier,” whereby ammonia fuel can be used “to generate H2 under mild conditions.”
Although ammonia (NH3) is made on a vast scale for use in fertilizers, its use as a chemical feedstock or as an energy carrier is much more limited. Many reactions that occur easily with its substitution products (amines) are sluggish for NH3, in part because of the difficulty of activating the N-H bond …
Amine-containing organic molecules are used in pharmaceutical and materials applications, and accessing these structures directly from ammonia could limit the generation of by-products during their synthesis. Bringing NH3 up to speed for these applications will require both the development of catalysts that can activate the strong N–H bond of ammonia and a fundamental understanding of the N–H bond cleavage step …
Bezdek et al report a molybdenum complex capable of weakening the N–H bond of NH3 and releasing a H atom to generate H2 under mild conditions.
Science, Ammonia activation at a metal, Jessica Hoover, 11/11/2016
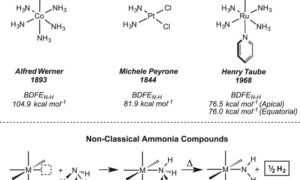
Specifically, the research, which comes out of The Chirik Group at the Chemistry Department of Princeton University, describes how the “coordination of ammonia … to a molybdenum complex substantially weakens the N–H … bonds, so much so that heating to 60°C liberates hydrogen.”
The development here is that, while there has been much research into ammonia’s “acid-base chemistry at room temperature, revolving around proton exchange … radical chemistry involving H-atom exchange is comparatively rare in these molecules in the absence of a high-energy stimulus.”
Here we report the synthesis of a molybdenum ammonia complex supported by terpyridine and phosphine ligands that lowers the nitrogen-hydrogen bond dissociation free energy from 99.5 (gas phase) to an experimentally measured value of 45.8 kilocalories per mole (agreeing closely with a value of 45.1 kilocalories per mole calculated by density functional theory). This bond weakening enables spontaneous dihydrogen evolution upon gentle heating …
Science, Coordination-induced weakening of ammonia, water, and hydrazine X–H bonds in a molybdenum complex, Bezdek et al, 11/11/2016
You can find the Princeton paper here, as well as the accompanying Perspectives piece by a researcher from West Virginia University – both articles are behind the Science paywall. The phys.org story is here.