Future Ammonia Technologies: Electrochemical (part 3)
By Trevor Brown on December 28, 2017
This series of articles on the future of ammonia synthesis began with a report on the NH3 Energy+ conference presentation by Grigorii Soloveichik, Program Director at the US Department of Energy’s ARPA-E, who categorized next-generation technologies as being either improvements on Haber-Bosch or electrochemical (with exceptions).
ARPA-E invests in “transformational, high-risk, early-stage research,” and recently began funding ammonia synthesis technologies, not to make renewable fertilizer but to produce “energy-dense zero-carbon liquid fuel.” This article will introduce the six electrochemical technologies currently in development with funding from ARPA-E.
ARPA-E is funding most of these through its REFUEL Program, but it is also funding some through its Open solicitations. While most of these projects were not ready to present results at the NH3 Energy+ conference in November, they each introduced their innovations and objectives at the REFUEL Kickoff Meeting that ARPA-E hosted in August.
In brief, Soloveichik sees the promise of electrochemical technologies relative to thermochemical ones (Haber-Bosch) in four major areas:
- “higher efficiency” allowing “energy savings”
- “higher selectivity” meaning “less purification needed”
- lower temperatures and pressures leading to reduced capex costs through “lower BOP” [balance of plant]
- “linear scalability” making these plants “better suited for small to medium scale.”
Direct electrochemical ammonia synthesis from nitrogen and water has a potential for big energy savings due to fewer process steps and a reduction in pressure / temperature. In addition, electrochemical technologies are easily scaled down to allow for low capital, modular plants that match the scale of renewable energy production. The REFUEL program’s very challenging target for the electrochemical process is 60% energy efficiency at a current density of >300 mA cm-2 and 90% coulombic efficiency.
Grigorii Soloveichik, ARPA-E, Future of Ammonia Production: Improvement of Haber-Bosch Process or Electrochemical Synthesis? November 2017
If there’s one thing that links the six projects that follow, it is that they illustrate the breadth and depth of the electrochemical field. Each one innovates beyond the current state of the art, but all are technologically distinct. Each one has the potential to enable renewable ammonia production and, in more practical terms, each one aims to deliver to ARPA-E a working bench-scale prototype in the very near future.
Ceramatec: Electrochemical Ammonia Synthesis for Grid Scale Energy Storage
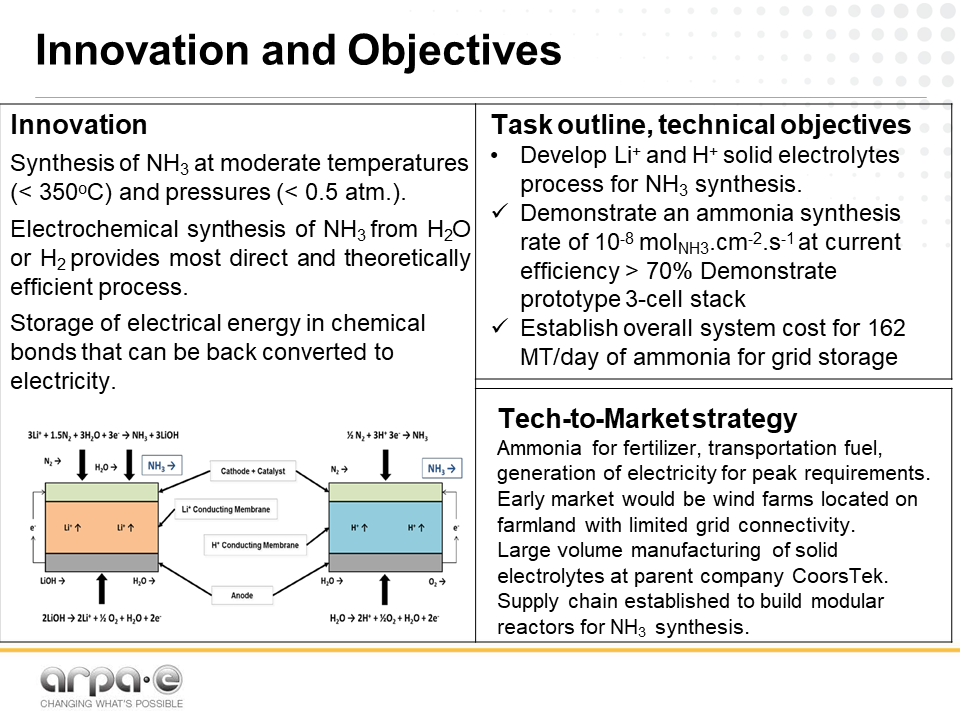
Ceramatec, the advanced ceramics manufacturer, has been quietly developing its solid electrolyte membrane technology, in partnership with the University of New Mexico and Los Alamos National Lab, since ARPA-E awarded funding in the 2015 Open Solicitation. In 2016, the team presented its initial results at the NH3 Fuel Conference in Los Angeles, CA.
The system, which combines Ceramatec’s “Li+ membrane process” with LANL’s “H+ exchange membrane/catalyst,” is designed to operate at “moderate temperatures (<350 °C) and pressures (<0.5 atm.).” The team hopes to produce ammonia at an energy consumption of 7.5 MWh per ton and, as one of its ARPA-E deliverables, to “establish overall system cost for 162 MT/day [metric ton per day] of ammonia for grid storage.”
This would be great for Ceramatec’s parent company, CoorsTek, which would handle the “large volume manufacturing of solid electrolytes,” establishing a supply chain for the modular reactors.
FuelCell Energy: Protonic Ceramics for Energy Storage and Electricity Generation with Ammonia
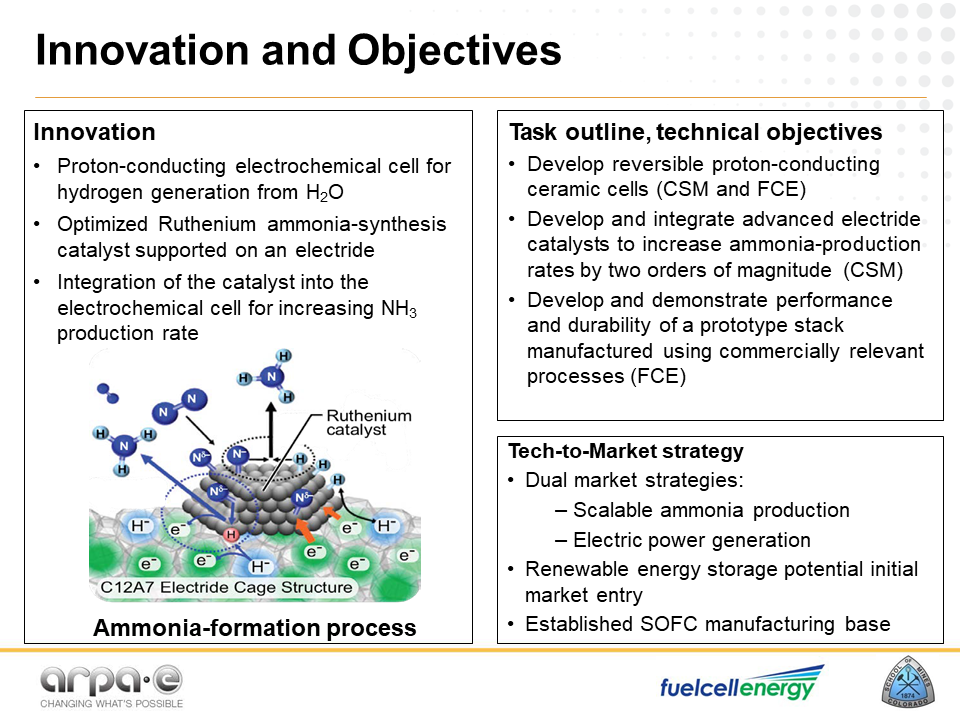
FuelCell Energy’s protonic ceramics ammonia technology is also an example of advanced ceramics, but this project focuses on “the integration of proton-conducting ceramic membranes with new electride catalyst supports [… which] increases the rate of ammonia formation by reducing coverage of the catalyst surface by hydrogen and allowing the nitrogen to use all of the catalyst area for reactions.”
According to their presentation at the REFUEL Kickoff Meeting, the catalyst support (“C12:A7”) has a unique “cage-like” structure that allows electron transfers “through the cages.” The result of this could be a “10x improvement in NH3 formation compared to Haber-Bosch catalyst.”
Wichita State University: Alkaline Membrane-Based Ammonia Electrosynthesis with High Efficiency for Renewable and Scalable Liquid-Fuel Production
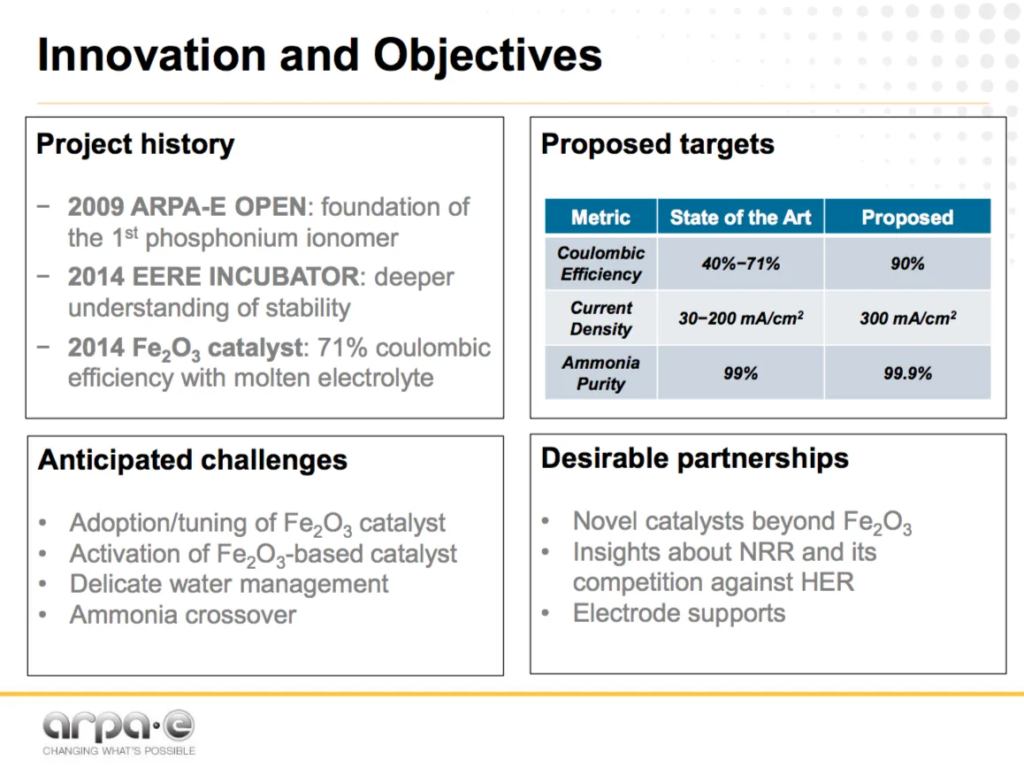
Wichita State University is working with George Washington University and Iowa State University to develop an alkaline membrane-based ammonia electrosynthesis technology, using a hydroxide exchange membrane (HEM) designed to operate at ambient temperature.
As the ARPA-E website slick-sheet explains: “the key innovation is the use of a hydroxide-exchange membrane (HEM) polymer electrolyte. The more commonly used proton exchange membranes (PEM) present major challenges leading to low efficiency for PEM-based ammonia electrosynthesis. Switching to HEMs will reduce side-reactions, allow the use of non-precious metal catalysts, and eliminate ammonia crossover and electrolyte contamination. As such, HEM-supported ammonia electrosynthesis may offer high coulombic efficiency and high ammonia productivity, without losing the key advantages of PEM-based electrosynthesis – operating under ambient conditions and using air and water as reactants.”
StoraGENergy: High Rate Ammonia Synthesis by Intermediate Temperature Solid-State Alkaline Electrolyzer
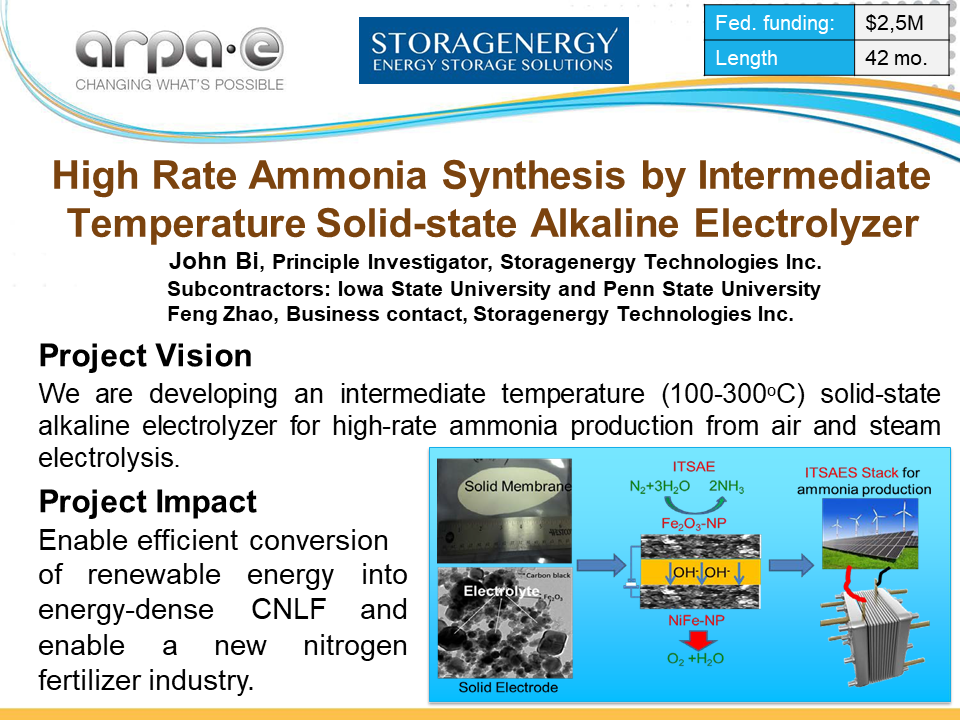
Storagenergy Technologies is also developing a solid state alkaline electrolyzer technology, in partnership with Iowa State University and Penn State University.
However, this system will be designed to operate at intermediate temperature (100-300 °C).
The electrochemical cell design will include a “nanostructured cathode catalyst, and a noble metal-free nanoparticle catalyst on the anode.”
Molecule Works: Novel Electrochemical Membrane Reactor
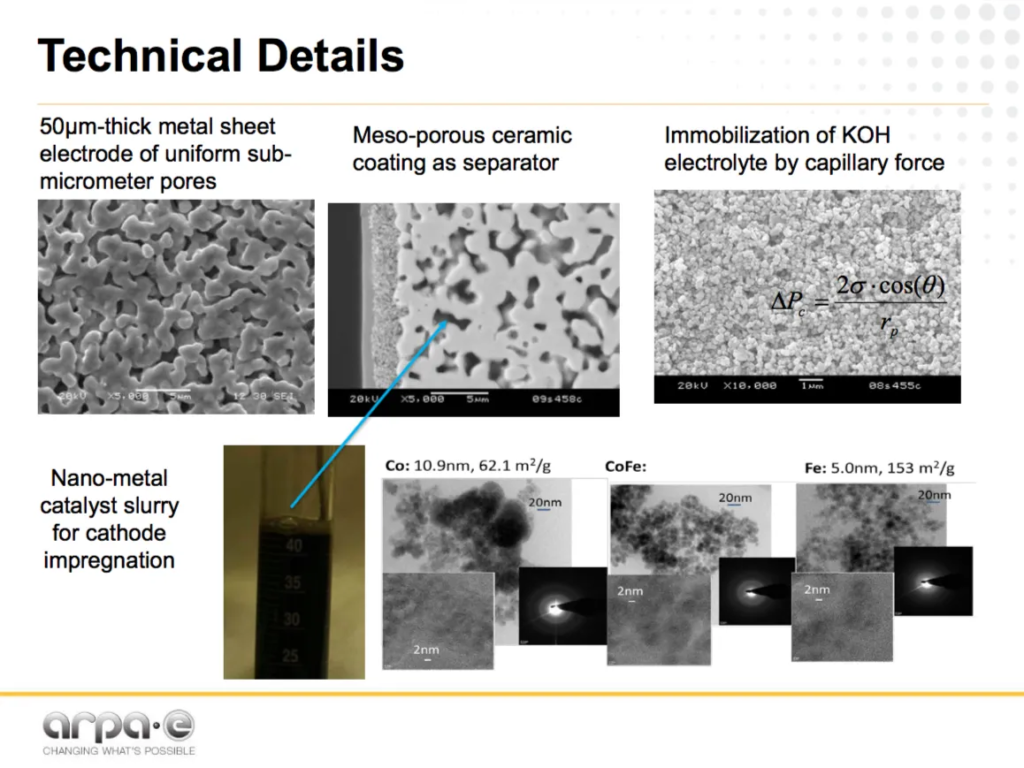
Molecule Works is developing its electrochemical membrane technology in partnership with Pacific Northwest National Laboratory (PNNL) and the University of Delaware. (U-Delaware is, separately, leading a REFUEL project to generate power from green ammonia using direct ammonia fuel cells).
This project combines advanced ceramics with metal membranes and nano-scale catalyst design. According to the presentation at the REFUEL Kickoff Meeting, the innovations here include:
- KOH electrolyte immobilized in porous ceramic membrane electrode assembly (MEA) for solid-state cell operation from 50-180 °C.
- Nano-catalyst incorporated into porous metal sheet cathode of high surface area.
Giner: High-Efficiency Ammonia Production from Water and Nitrogen
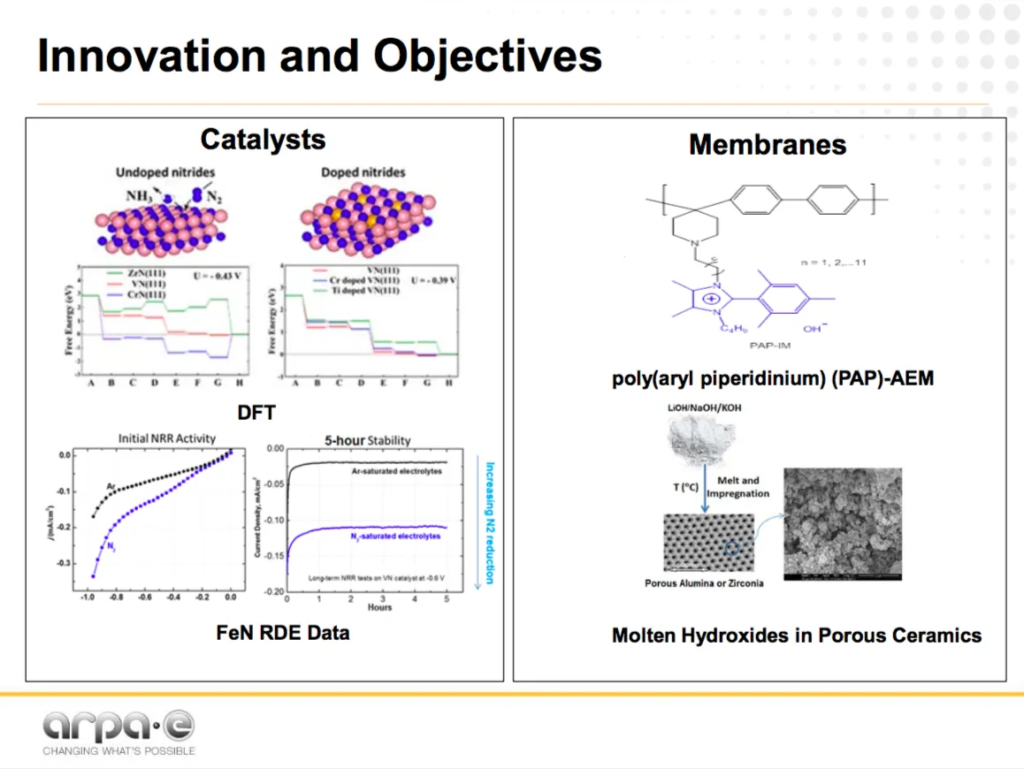
Giner is developing an anion exchange membrane technology, using metal nitride catalysts at “high temperature … (>100 °C).”
In this project, “poly(aryl piperidinium) anion exchange membranes (AEM)” will “boost the ammonia production rate and enhance process stability.”
Like Ceramatec and its parent company, Giner is ready to benefit from this innovation commercially: the system will be designed so that it can be integrated into the company’s existing water electrolysis technology, allowing Giner “to maximize the overall system efficiency.”
If everything goes according to schedule, the first of these ARPA-E electrochemical projects will publish their results in 2018 and also, perhaps, deliver working bench-scale prototypes. If, in the meantime, you need more information on electrochemical ammonia synthesis technologies, see Part 1 and Part 2 of this series.
You can also read the full article at AmmoniaIndustry.com.