N-Fuels vs. C-Fuels: Nitrogen “superior” to carbon as a hydrogen carrier
By Stephen H. Crolius on November 17, 2017
Gideon Grader, a Faculty Dean at Technion Israel Institute of Technology, and Bar Mosevitzky, one of the members of his laboratory, spoke in separate talks at the NH3 Energy + Topical Conference about one of the Grader Research Group’s key focuses: nitrogen-based energy carriers. Grader and his team champion the idea that ammonia can be the starting rather than ending point for nitrogen-containing fuels for heat engines. The focuses of their research include ammonium hydroxide ammonium nitrate (AAN), ammonium hydroxide urea (AHU), and urea ammonium nitrate (UAN). As described below, this work is an indispensable addition to the C-fuel vs. N-fuel debate well known to proponents of ammonia energy. And the Grader team takes a position: per the abstract of Grader’s talk, “using nitrogen as a hydrogen carrier can potentially offer a superior option.”
Grader’s talk provided summaries of work in three areas pertinent to the profiling of a potential fuel: energy balance, autoignition behavior, and exhaust aftertreatment. Mosevitzky’s talk delved deeply into thermal autoignition of AAN.
To facilitate comparison of energy balance across different renewably derived fuels, the Grader team developed the power-to-fuel-to-power (PFP) index. The premise is that the harvest of energy from sustainable primary sources like the sun and wind will first involve conversion to electricity and then conversion to hydrogen as a commodity that is more readily stored than electricity itself. So far so good, but many stakeholders recognize that one more conversion is desirable. In Grader’s words, “storing hydrogen on carrier atoms provides a safe and convenient way to utilize and transport renewable energies.” The question is whether carbon or nitrogen should serve as the carrier atom: C-fuel vs. N-fuel. The PFP index offers quantitative substantiation of the Grader team’s suggestion that N-fuel may ultimately win the race. (Note: The PFP index has been mentioned in Ammonia Energy on two previous occasions: March 2, 2017 and April 20, 2017. The paper originally detailing the PFP index was published on June 10, 2016 in Angewandte Chemie.)
Grader’s team calculated a PFP index number for seven fuel species. Three are among the simplest carbohydrates that can be made by reacting carbon dioxide with hydrogen: methane, methanol, and dimethyl ether. On the nitrogen side, the team includes ammonia and the three derivatives mentioned above (AAN, AHU, and UAN). All three of the latter include water in their formulation.
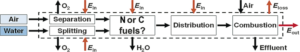
The PFP index represents the ratio between the energy cost of synthesizing a fuel and the value of the energy embodied in the fuel. The outcomes of the calculations are shown for each fuel species in a table that breaks down energy inputs into air separation, hydrogen generation, chemical synthesis, and transport to market. The efficiency of energy extraction in the heat engine is also considered.

Of the seven fuels analyzed, ammonia is shown to have the highest PFP index. Its score of 35% is significantly higher than the indices for methane (31%), methanol (32%), and DME (28%) when these are produced using CO2 from a flue stream. The key factor driving the deltas between ammonia and the C-fuels is the energetic cost of capturing CO2 vs. that of capturing nitrogen. This result is even more dramatic for direct air capture (DAC) of CO2. In this case, the PFP indices for the carbon-based molecules fall to 27%, 27%, and 23%, respectively.
These findings are encouraging for ammonia energy proponents and should also be of interest to stakeholders within the emerging CO2-capture industry. A number of firms, including Carbon Recycling International, Climeworks, and Carbon Engineering have sprung up over the last decade with enhanced technology for extracting CO2 from flue streams and/or the air. In formulating their business plans, the companies naturally wonder about the revenue stream that could come from producing fuels. Carbon Engineering, for example, articulates its business model in this fashion: “Air To Fuels is our technology that uses atmospheric CO₂, captured using our DAC process, and combines it with renewably produced hydrogen, to directly synthesize . . . hydrocarbon fuels such as diesel, gasoline, and Jet-A. Air to fuels gives us a way to produce global scale quantities of fuels that are compatible with today’s transportation infrastructure and engines . . .”
This is an appealing vision but it seems little has been published on the economics of the proposition, especially relative to the economics of nitrogen-based fuels. As it happens, just two weeks before the NH3 Energy + Conference, the Journal of Energy Security published a paper entitled “Shifting the paradigm: Synthetic liquid fuels offer vehicle for monetizing wind and solar energy.” The paper’s author is Maxim Lyubovsky, an ORISE Fellow at the Fuel Cell Technologies Office at the U.S. Department of Energy. Among many other things, the Fuel Cell Technologies Office is the home of the United States’ H2@Scale initiative.
In the paper, Lyubovsky analyzes liquid fuels that could potentially contribute to a hydrogen-based sustainable energy economy. The species he considers include methanol, ethanol, and longer-chain hydrocarbons that could be produced via Fischer Tropsch synthesis. The long-term carbon feedstock he envisions is CO2, captured directly from the atmosphere.
Lyubovsky acknowledges that DAC is costly for a molecule that is present in the atmosphere at 400 ppm, but argues that the energy needed to separate CO2 from the air “should be compared with the energy required for the production of hydrogen.” He implies that, because the energy used for separation is a small fraction of the energy used to produce hydrogen, any cost disadvantage in the separation step “can be successfully overcome if there is sufficient market drive.”
The Grader team’s PFP index shines a helpful light on this question. When the complete set of numbers is marshalled, it can be seen that the energy disadvantage of capturing a species present in the atmosphere at 400 ppm (CO2) is enormous relative to capturing a species present at 780,000 ppm (N2) — 18.1 GJ per tonne for methane vs. 0.18 GJ per tonne for ammonia. The size of the gap is indeed smaller than the amount of energy consumed in producing hydrogen, but this reality does not make the gap go away. Rather, the gap persists and is present in the final reckoning: a PFP index of 35% for ammonia and 27% for methane via DAC.
In another section of his paper Lyubovsky writes, “one disadvantage of ammonia as an energy carrier, outside the need for elevated pressure to keep it in liquid form, is that its use as fuel would require building essentially new infrastructure.”
The Grader team’s broader work has great relevance for this question. Let’s say for the sake of argument that “the need for elevated pressure” is a legitimate hurdle, even though propane’s need for the same level of elevated pressure has not foreclosed its widespread use as an energy commodity and transportation fuel. Grader’s ammonia-derived fuels clear the hurdle because they can be managed, economically and safely, as liquids at atmospheric pressure and ambient temperatures. These fuels could thus be handled by infrastructure whose design is similar to that used in today’s liquids-oriented energy system.
And then, if the design could be similar, what might prevent the actual assets in place today from being repurposed for nitrogen-based fuels? The definitive answer to this question does not yet exist. But it can be observed that ammonia and its derivatives are currently produced, transported, and consumed at volumes of hundreds of thousands of tonnes daily. The infrastructure that supports this activity is built from steel, as is the case for the major energy commodities currently in use. It is perhaps this materials commonality that prompts Mosevitzky to state in his presentation that AAN is “compatible with current fuel infrastructure.”
However, according to Grader’s figures, there is a tradeoff in moving from ammonia to its derivatives. While ammonia itself has an energy density that is 95% of methanol’s, AHU’s is only 40%, while AAN’s and UAN’s are only 16% and 14% respectively. Time and the marketplace will sort out the relative economic implications of energy balance and energy density. But the work of Grader and his colleagues will expedite the sortation process, and increase the chances that it culminates in well-informed conclusions.