The Case for Giving Direct Ammonia Fuels Cells a Shot
By Stephen H. Crolius on August 22, 2019
The Center for Catalytic Science and Technology (CCST) at the University of Delaware has made new strides in the development of a direct ammonia fuel cell (DAFC) suitable for use in transportation applications. The progress is reported in “An Efficient Direct Ammonia Fuel Cell for Affordable Carbon-Neutral Transportation,” a paper published last month by Yun Zhao and six coauthors in the journal Joule. The paper gives an impressive account of CCST’s technical advances; and it makes a distinctly compelling case for the relevance of CCST’s work on DAFCs. In the latter regard, the authors find that ammonia has the “lowest source-to-tank energy cost by a significant margin” relative to other fuels that can be derived from renewably generated electricity. It is in society’s interest, they strongly imply, to give DAFC technology a chance to compete with hydrogen-based fuel cells in automotive applications.
Evaluating Candidate Fuels

The paper’s launch point is the challenge of decarbonizing the transportation sector. In the authors’ view, both battery electric architectures and biofuels will make important contributions. But in the first case, “most air transport, military, shipping, and long-distance freight applications remain challenging for batteries because of their limited energy density. Even for consumer light-duty vehicles, particularly larger size passenger vehicles, limited access to charging infrastructure and/or the substantial recharge time may drive continued demand for alternatives.” In the second case, while “biofuels replicate most of the desirable properties of petroleum-based fuels . . . the scale of biofuels is limited by their environmental footprint, considering the land and water usage.” Hence, one or more additional carbon-neutral fuels will be needed.
The authors group the alternatives into three categories: hydrogen, carbon-based fuels, and nitrogen-based fuels. On the carbon side, the candidate list includes methanol, dimethyl ether, and molecules created with Fischer Tropsch technology. Ammonia, urea, and hydrazine are the contenders on the nitrogen side. Hydrogen is evaluated only in gaseous form. After surveying previous technoeconomic analyses of these fuels, the authors report that “none compare nitrogen-based fuels, carbon-based fuels, and hydrogen under a unified set of assumptions.” Having thus identified a gap, they develop a comprehensive framework to fill it.
Source-to-tank energy costs of hydrogen, ammonia, and methanol were compared considering the cost of hydrogen production from renewable electricity integrated with the grid, geological hydrogen storage, air separation and fuel production from hydrogen (for ammonia and methanol), long-distance transmission (by pipeline), distribution to refueling stations (by truck), and dispensation at the station.
Zhao et al., An Efficient Direct Ammonia Fuel Cell for Affordable Carbon-Neutral Transportation, Joule (2019)
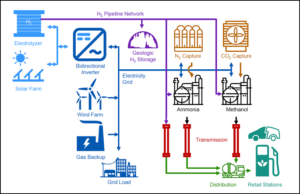
The authors mention two interesting findings on their way to cost-per-unit numbers. The first is that ”continuous operation with geological hydrogen storage . . . was found to be much cheaper, even without considering the operational difficulties of . . . intermittent operation of the chemical plants.” (Reference is made to Geologic storage of hydrogen: Scaling up to meet city transportation demands, a 2014 paper that draws encouraging though preliminary conclusions about the feasibility and costs of a network of city-specific geologic storage facilities.)
The second is that the CO2 feedstock for carbon-based fuels must come from direct air capture rather than extraction from flue gases of conventional power plants and industrial establishments. On the one hand, the authors say, direct air capture “represents a carbon-neutral pathway, without requiring assignment of carbon dioxide emissions between two processes (carbon-producing process and carbon-emitting vehicle).” On the other, “in a scenario where transportation fossil fuels are being replaced with carbon-neutral fuels, most energy-related flue gas sources could be eliminated through electrification or substitution of hydrogen, leaving only a much smaller and more dispersed pool of process emissions to draw from.”
When the number-crunching is complete, “hydrogen, ammonia, and methanol are identified as the most cost-effective candidates among hydrogen-, nitrogen-, and carbon-based fuels, respectively.” Quantitatively, “on a source-to-tank basis, ammonia costs ($4.50 GGE-1) are 31% lower than hydrogen ($6.55 GGE-1) and 18% lower than methanol ($5.46 GGE-1).” The key drivers of hydrogen’s cost position are “high transmission, distribution, and dispensation costs (totaling $4.16 GGE-1) as a compressed gas.” For methanol, the critical factor is “the high cost of separating carbon dioxide from air ($195 t-1).”
The cost of the fuel as it dispensed into a vehicle is certainly of interest to a driver, but the bottom-line parameter is fuel cost per kilometer traveled. Based on fuel efficiency values from Argonne National Laboratory’s Greenhouse Gases, Regulated Emissions, and Energy Use in Transportation (GREET) model, the authors calculate the cost for hydrogen as $0.072 per kilometer and estimate the cost for methanol as $0.091 per kilometer. Embedded in the hydrogen cost is a “peak system efficiency” value of 60%. To beat $0.072 per kilometer and take the lead in this race, the authors calculate that a DAFC-powered vehicle “must achieve a peak system efficiency of 42%.”
Technical Advances
The authors make clear that their technical advances represent improvements that are somewhere between important and indispensable to attainment of the system efficiency goal. Specifically, they have identified “two key factors . . . for achieving high DAFC performance: the use of a PGM-free cathode catalyst of zero AOR activity and a HEM which is highly stable at 80 degrees C or higher.” (PGM stands for the notoriously expensive platinum group metals. AOR stands for ammonia oxidation reaction, which sounds like a good thing for an ammonia fuel cell but is bad if it happens in the wrong place. HEM stands for hydroxide exchange membrane, the structure at the heart of the fuel cell.)
These factors relate directly to parameters central to fuel cell viability in an automotive context. One such parameter is fuel cell acquisition cost, which can be significantly influenced by the cost of catalysts, especially those containing PGMs. In a December 2018 paper, “The Direct Ammonia Fuel Cell and a Common Pattern of Electrocatalytic Processes,” CCST faculty member Shimshon Gottesfeld estimated that the $30/kW cost target for an automotive fuel cell stack could be met with PGM loading of 0.1 grams of platinum per kW of fuel cell power capacity. In the Joule paper, the authors report that they have developed a catalyst that foregoes the inclusion of PGMs altogether.
Another make-or-break parameter is durability, with specific reference to the negative correlation typically observable between increasing operating temperature and electrolyte membrane useful life. In the Joule paper, the authors report that they have identified an HEM that exhibits excellent durability at operating temperatures of 80 degrees C.
The yardstick to measure the ultimate impact of the CCST advances is fuel cell power density, measured in milliwatts (mW) per square centimeter of membrane area. Gottesfeld estimated that 500 mW/cm2 would be the minimum threshold of relevance for DAFCs. The Joule authors mention that Gottesfeld had achieved 420 mW/cm2 in his experimental set-up. With their particular configuration of PGM-free catalyst and durable membrane, the Joule authors report that they have achieved 135 mW/cm2 at 80 degrees C.
Do the authors think they will be able to reach the threshold of relevance? They do not say. But they create the impression of momentum.
Click here for Ammonia Energy’s previous reporting on the University of Delaware’s DAFC program.