The Future of Ammonia: Improvement of Haber-Bosch … or Electrochemical Synthesis?
By Trevor Brown on November 17, 2017
During our NH3 Energy+ Topical Conference, hosted within AIChE’s Annual Meeting earlier this month, an entire day of presentations was devoted to new technologies to make industrial ammonia production more sustainable.
One speaker perfectly articulated the broad investment drivers, technology trends, and recent R&D achievements in this area: the US Department of Energy’s ARPA-E Program Director, Grigorii Soloveichik, who posed this question regarding the future of ammonia production: “Improvement of Haber-Bosch Process or Electrochemical Synthesis?”
The future of ammonia production
Soloveichik looks at ammonia not just as a fertilizer but also as a hydrogen carrier, with market potential in renewable energy storage and distribution, as a carbon-free fuel. The energy market places value on the three hydrogen atoms of NH3, whereas the fertilizer market values only its one nitrogen atom.
It is important to acknowledge this new potential market for ammonia because the vast scale of the energy market completely alters the investment drivers and investment horizon for sustainable ammonia production technologies: we are talking about a future energy market that is orders-of-magnitude larger than today’s fertilizer market.
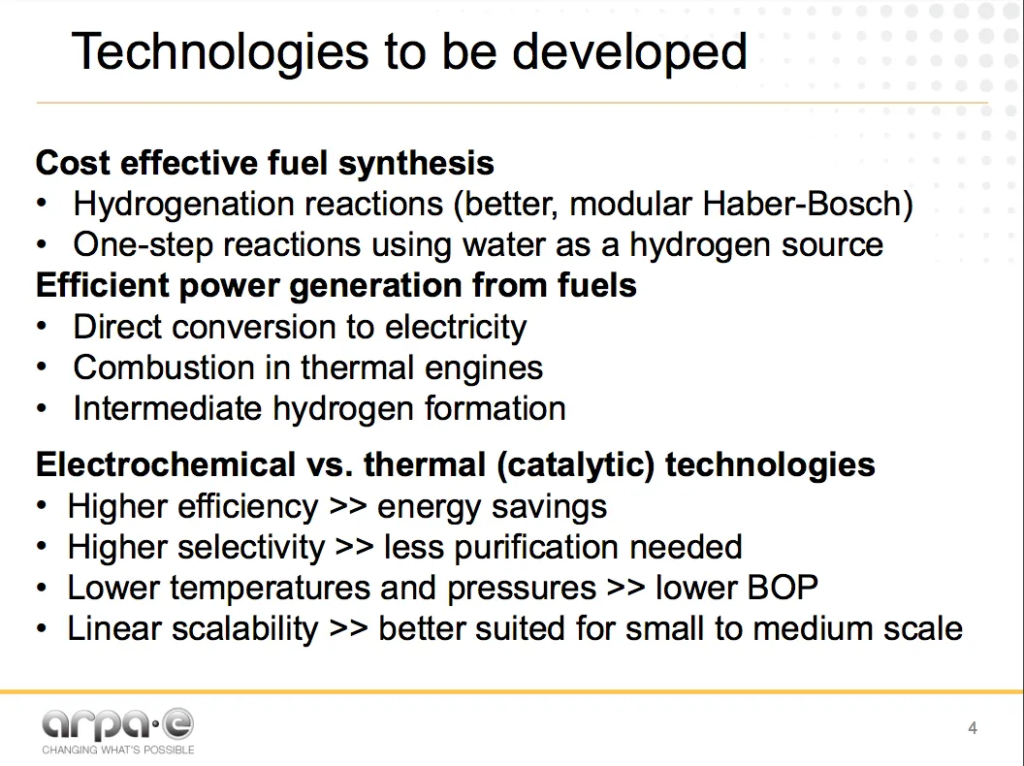
Soloveichik’s overview of “Technologies to be developed” includes incremental improvements on the current Haber-Bosch process as well as brand new processes using electrochemical and chemical reactions.
There are essentially four drivers behind Soloveichik’s technological wishlist:
- Sustainability: using water as a hydrogen source instead of natural gas or other fossil fuels, eliminating carbon emissions.
- Energy efficiency: using less energy (fuel + feedstock) to produce the same quantity of ammonia.
- Modular scalability: sizing the industrial process to be optimized for renewable energy inputs rather than fossil energy inputs.
- Economic viability: reducing the capital and operating costs of any new system so that it can compete with the incumbent technology.
Today’s Haber-Bosch
The energy intensity and vast scale necessary for economic operations of the Haber-Bosch process are two well-known challenges of the fertilizer industry. For example, it is now almost impossible to de-risk the $3 billion investment necessary to build (or, more specifically, to finance) a new fossil-ammonia plant in North America: it is just too big.
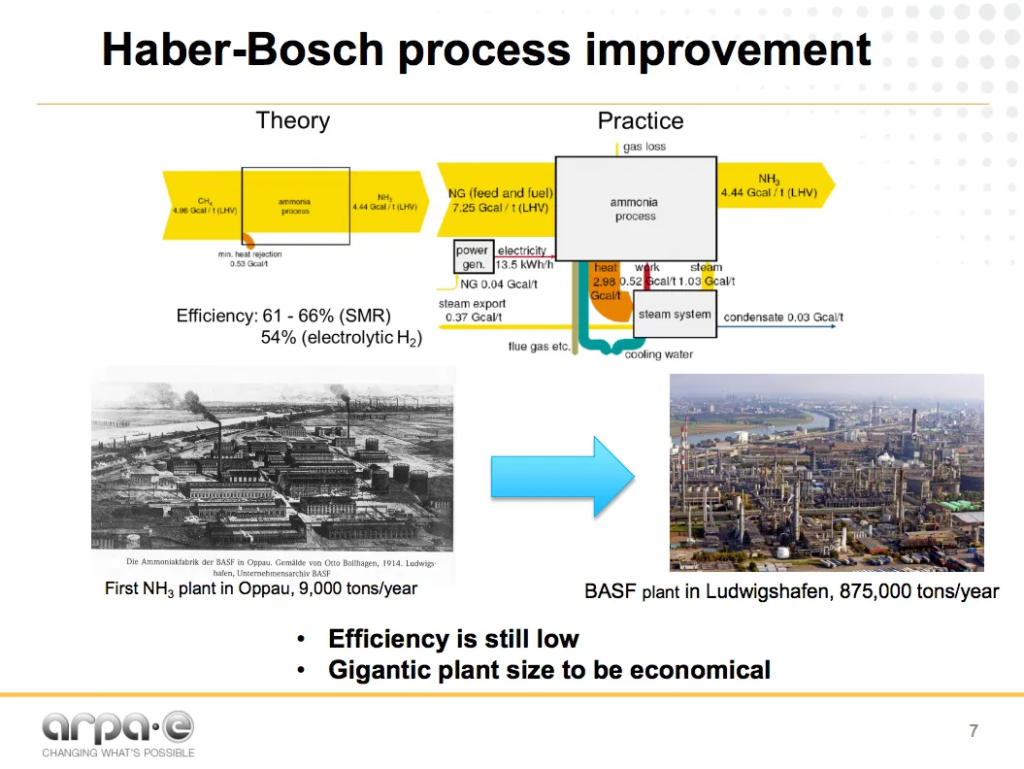
And while the fertilizer industry is justifiably proud of its recent energy efficiency improvements, it occasionally makes excuses for itself: citing how close the latest ammonia plants are to the technological limits of the Haber-Bosch process.
Soloveichik is attempting to address both of these downsides: energy intensity and vast scale of economy.
As his energy-flow diagram makes clear, being close to the technological limit of an energy intensive process doesn’t make Haber-Bosch an energy efficient process. He demonstrates that steam methane reformation (SMR) Haber-Bosch is 61-66% efficient, at best. This compares to an older technology for hydrogen production, water electrolysis, which is very close, at 54% efficient, with no carbon emissions and no fossil fuel reliance.
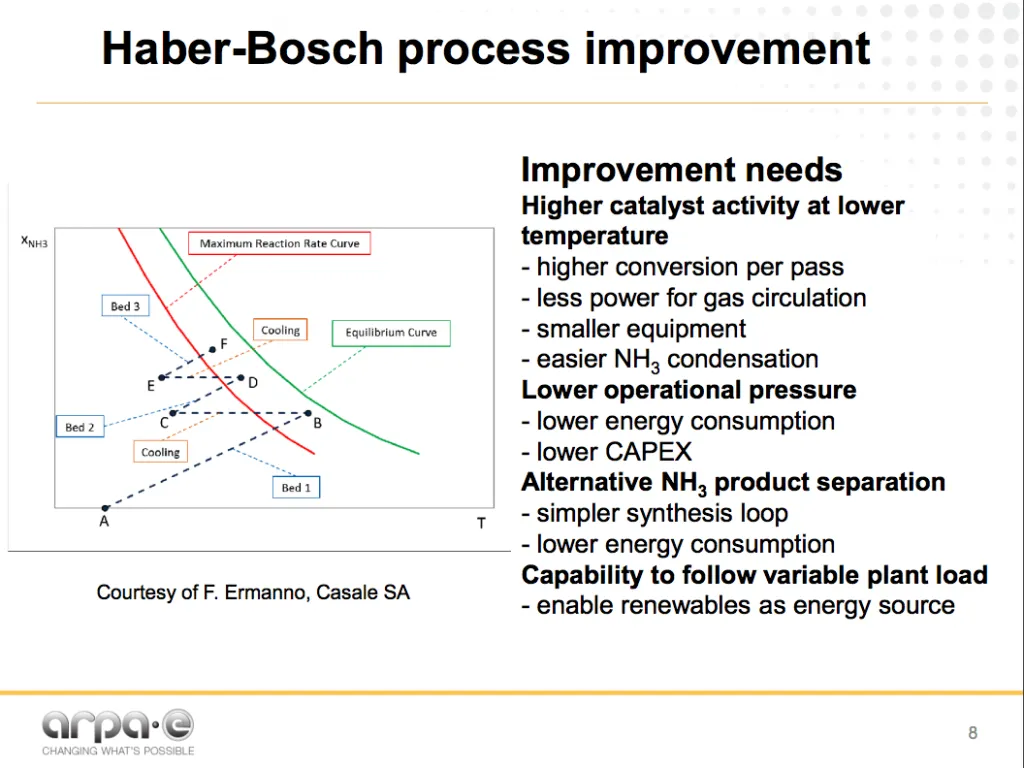
The next generation of Haber-Bosch
Soloveichik understands the limitations and the opportunities inherent in the existing technology. His REFUEL program at ARPA-E is currently funding over a dozen new ammonia synthesis and fuel-use projects, pursuing both novel electrochemical routes and incremental improvements for ammonia production.
The next generation of Haber-Bosch chemistry should work at lower pressure and temperature, which can be achieved by using more active catalysts or a combination of catalysts with physical activation, and by continuous ammonia removal from the reaction zone thus shifting the equilibrium. To make it competitive with conventional process, the process efficiency should be >86% and the catalyst should provide a reaction rate of at least 7×10-7 mol cm-2 s-1.
Grigorii Soloveichik, ARPA-E, Future of Ammonia Production: Improvement of Haber-Bosch Process or Electrochemical Synthesis? 11/01/2017
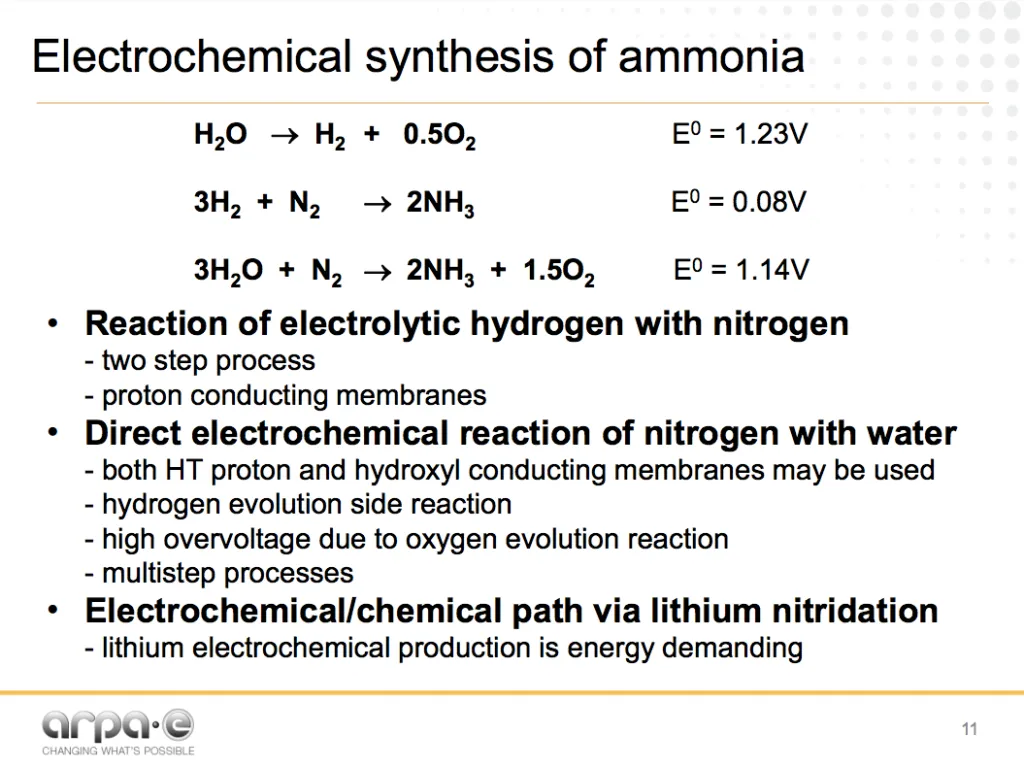
Electrochemical ammonia synthesis
I will go into greater detail next week on the individual technologies being developed under the REFUEL program, as well as other electrochemical technologies presented at the NH3 Energy+ Conference.
However, the primary appeal of electrochemical technologies remains unchanged: “Potentially lower energy consumption.”
Direct electrochemical ammonia synthesis from nitrogen and water has a potential for big energy savings due to fewer process steps and a reduction in pressure / temperature. In addition, electrochemical technologies are easily scaled down to allow for low capital, modular plants that match the scale of renewable energy production. The REFUEL program’s very challenging target for the electrochemical process is 60% energy efficiency at a current density of >300 mA cm-2 and 90% coulombic efficiency.
Grigorii Soloveichik, ARPA-E, Future of Ammonia Production: Improvement of Haber-Bosch Process or Electrochemical Synthesis? 11/01/2017
We should begin to see the tangible outcomes of the REFUEL project (lab-scale demonstration data) within the next year. If you wish to know more in the meantime, the presentations from ARPA-E’s REFUEL Kickoff Event are now available to download from the Department of Energy website.
In his NH3 Energy+ presentation, Soloveichik described incremental improvements on the Haber-Bosch process as the “low hanging fruit” for sustainable ammonia.
This is exactly how the International Energy Agency’s Cedric Philibert described sustainable ammonia in his new report published last week, saying “Renewables-based electrolysis of water can produce hydrogen-rich chemicals such as ammonia or methanol … Such low electricity prices could allow hydrogen to be produced at costs competitive with natural gas reforming, oil-cracking or coal gasification – without CO2 emissions. The low hanging fruit seems to be ammonia.”
You can also read the full article at AmmoniaIndustry.com.