Ontario Tech Develops Its Own Flavor of Direct Ammonia Fuel Cell
By Stephen H. Crolius on December 19, 2019
Fuel cells are widely expected to play a major role in the sustainable energy economy of the future: because they manifest a high level of energy efficiency relative to incumbent energy conversion devices, and because they are a good match for hydrogen as a fuel species. The size of their role will ultimately be determined by an encompassing economic calculus that considers, most crucially, system cap ex, fuel cost, and conversion efficiency. Beneath each of these factors is a host of characteristics that contribute to economic outcomes in large and small ways. The characteristic of simplicity, though, has a uniquely intuitive appeal. Doesn’t the simplest approach often carry the day: because it has fewer parts and/or steps, and because there is less that can go wrong with it?
These thoughts came to mind during perusal of a paper that appeared in the June 2019 edition of the journal Chemical Engineering Science. The paper, “Development and performance evaluation of a direct ammonia fuel cell stack,” was written by Osamah Siddiqui and Ibrahim Dincer, both active at the Clean Energy Research Laboratory at Ontario Tech University in Canada (formerly University of Ontario Institute of Technology). Their design may or may not ever reach the point of commercialization, but there is no denying its essential simplicity.
To bring this into focus, consider the range of fuel cells designed to run on ammonia. Appropriately configured solid oxide fuel cells (SOFCs) can accept ammonia directly as a fuel. Simple. But the high temperatures at which SOFCs operate introduce a constraint: they are best suited for applications that involve continuous or near-continuous operation. Low-temperature fuel cells, by contrast, can be applied to use cases involving short and frequent on-off cycles, as well as those involving continuous operation. But most of the designs leading the low-temperature pack involve front-end modules that convert ammonia into hydrogen. In some variants, the requirement is to achieve extreme hydrogen purity. In others, the demands on the front end are moderated by stack designs that can tolerate uncracked ammonia. Either way, a system with a front end is not as simple as a system that accepts ammonia directly. (Ammonia Energy summarized many of the pertinent development efforts in a 2018 Annual Review article.)
The design demonstrated by Siddiqui and Dincer does without a front end, and in fact does without almost everything else except the fuel cell stack itself. The stack includes the normal components that make up the anode-membrane-cathode sandwich. Gaseous ammonia, introduced on the anode side, flows through a gas diffusion layer coated with a platinum-containing catalyst, in the process undergoing the series of reactions that produce diatomic nitrogen, water, and free electrons. Humidified air, subject to a small degree of compression and introduced on the cathode side, flows through another gas diffusion layer which is also coated with a platinum-containing catalyst. The product here, with the addition of the electrons that were produced on the anode side, is hydroxide (OH-) ions. An anion exchange membrane selectively allows the passage of the hydroxide ions to the anode side, where they are central players in the ammonia-to-water reaction sequence. The set-up and electrochemistry are simplicity itself.
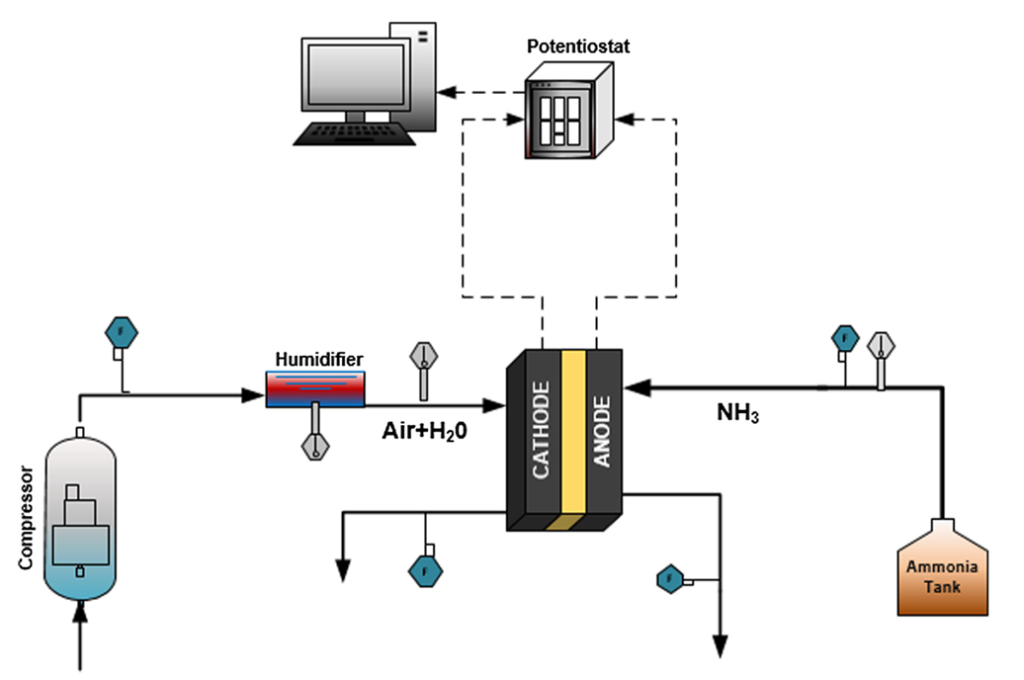
Siddiqui and Dincer report that their technology, in a five-cell array and with humidified air infused at 80 degrees C, has achieved a power density of 1.34 mW/cm2 +/- 0.04 mW/cm2. This is in contrast to the 135 mW/cm2 reported in a 2019 paper in the journal Joule by the direct ammonia fuel cell team at the University of Delaware, also using a platinum-containing catalyst at the anode (but not the cathode) and an operating temperature of 80 degrees C. (An Ammonia Energy article on the Joule paper appeared in August 2019.) A key difference between the Ontario and Delaware systems is that the Delaware group employs potassium hydroxide as a liquid electrolyte.
The disparity in power density may prove to be an insurmountable disadvantage for the Ontario technology, but Siddiqui and Dincer report an energetic efficiency for their device of 52.4%. (Dincer pointed out for Ammonia Energy’s benefit that this is based on the assumption of 100% coulombic efficiency “which implies that all ammonia that reacted resulted in the production of current … H2 PEM fuel cells available commercially that report efficiencies of nearly 50% are actual operating efficiencies based on the power output and fuel input. The efficiencies of such cells when evaluated with 100% coulombic efficiency assumption reach nearly 80%.”) The Joule paper does not report energy efficiency for the Delaware technology.
Dincer is a member of the Faculty of Engineering and Applied Science at Ontario Tech. After receiving his doctorate in 1993, he devoted himself to a variety of future-oriented energy topics including renewable energy systems and hydrogen fuel cells. His bibliography of papers related to ammonia energy goes back to at least 2011. (He reports that his interest was kindled in 2006.) Siddiqui is a Research Assistant at Ontario Tech and is the author of numerous hydrogen- and ammonia-themed papers, many with Dincer as co-author.
This article was updated on January 17, 2020 to reflect Ontario Tech University’s March 2019 name change.