Future Ammonia Technologies: Electrochemical (part 1)
By Trevor Brown on December 22, 2017
Last month’s NH3 Energy+ conference featured presentations on a great range of novel ammonia synthesis technologies, including improvements to Haber-Bosch, and plasmas, membranes, and redox cycles. But, in a mark of a conference approaching maturity, members of the audience had at least as much to contribute as the presenters.
This was the case for electrochemical synthesis technologies: while the presentations included updates from an influential industry-academia-government collaboration, led by Nel Hydrogen’s US subsidiary, the audience members represented, among others, the new electrochemical ammonia synthesis research lab at Massachusetts Institute of Technology (MIT), and a team from Monash University in Australia. The very next week, Monash published its latest results, reporting an electrochemical process that synthesized ammonia with 60% faradaic efficiency, an unprecedented rate of current conversion at ambient pressure and temperature.
Electrification and Decarbonization of the Chemical Industry
Karthish Manthiram recently established the Manthiram Lab at MIT to focus on “molecular engineering of electrocatalysts for clean energy technologies and synthesis of fine and commodity chemicals.” Sustainable ammonia is one of his starting points.
As Manthiram and co-author Zachary Schiffer put it in their recent article, Electrification and Decarbonization of the Chemical Industry, electrochemical processes “have some advantages over traditional thermochemical methods,” because electricity “offers an alternative driving force, voltage, that can enable operation at mild temperatures and pressures.”
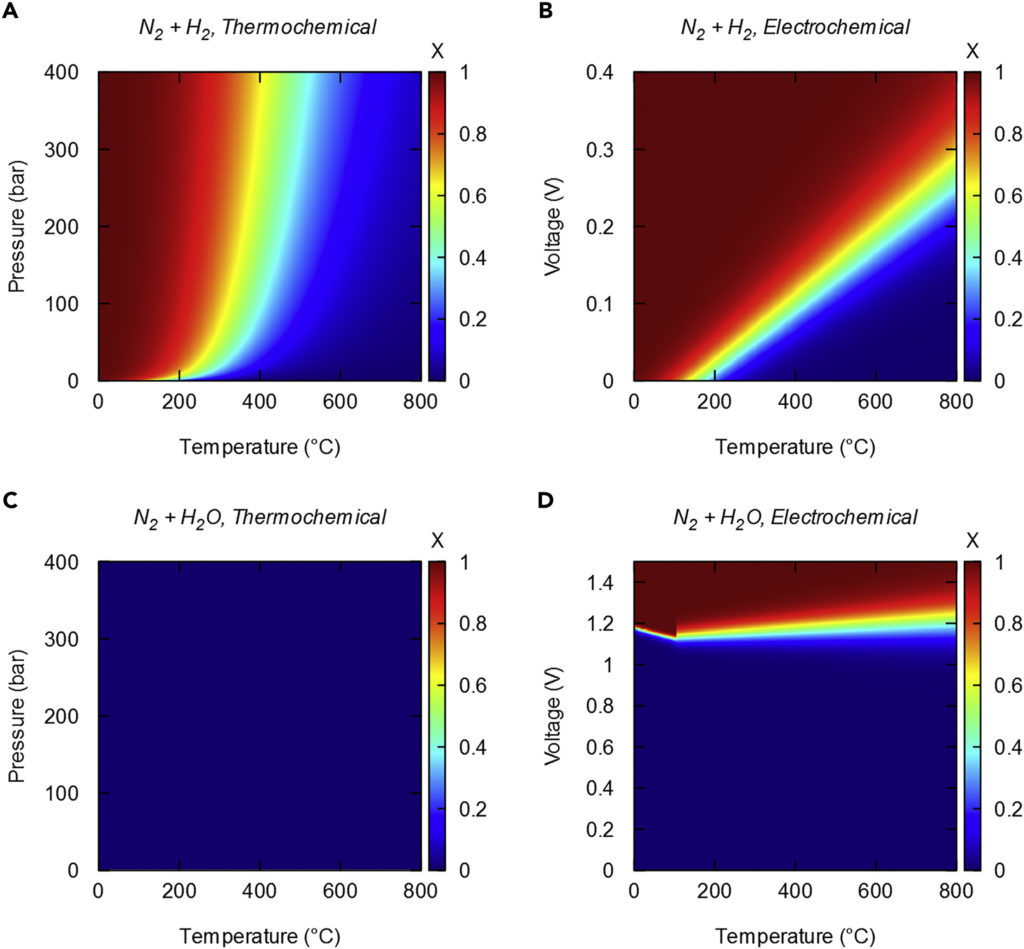
The article illustrates the potential impact of electrochemistry, relative to a thermochemical process like Haber-Bosch, with a series of colored charts that show the thermodynamics of the reactions, plotting temperature against pressure or voltage.
Red areas indicate where the conversion will be complete (all of the reactants will have been turned into product: meaning hydrogen and nitrogen will have reacted to produce ammonia). On the other hand, blue areas indicate conditions (temperature and pressure/voltage) where no conversion will occur.
Results for thermochemistry (Haber-Bosch) are on the left; results for electrochemistry are on the right. In addition, the top charts illustrate the reactants used in Haber-Bosch, hydrogen and nitrogen, but in the bottom charts the authors illustrate the use of simpler reactants, water and nitrogen. This demonstrates a key potential benefit of electrochemical ammonia synthesis: the avoidance of intermediate hydrogen production.
(A) The thermochemical Haber-Bosch process requires elevated pressures to compensate for the high temperatures needed to achieve fast kinetics in the reaction of nitrogen and hydrogen to produce ammonia.
(B) In an electrochemical reactor operating on nitrogen and hydrogen feedstocks, small voltages can be used to achieve high equilibrium conversions.
(C) Conversion of nitrogen and water to ammonia is negligible using temperature and pressure.
(D) Application of a suitable potential can drive the conversion of nitrogen and water to ammonia, demonstrating the promise of electrochemical routes for producing ammonia.
Schiffer and Manthiram, Electrification and Decarbonization of the Chemical Industry, September 2017
These charts illustrate the well-established argument for electrochemical ammonia synthesis: “the standard operating conditions for the Haber-Bosch process are around 450°C and 200 bar. Replacing pressure with voltage … the thermodynamics of the system become favorable without the use of elevated pressures; the voltage may also help to accelerate the kinetics.”
In market terms, this shift in technology would have a profound impact. Without high pressures and high temperatures, ammonia plants would no longer need to be “large-scale, centralized reactors to achieve economic viability.” This is because electrochemical production would be “conducive to modularity.”
Reactors can be much smaller and located alongside renewable electricity sources. By producing the ammonia close to where it is needed, distribution costs can be reduced … The modularity may open up the possibility of producing ammonia-based fertilizers locally, especially in parts of Africa where infrastructure for the production and distribution of fertilizers is lacking.
Schiffer and Manthiram, Electrification and Decarbonization of the Chemical Industry, September 2017
This echoes the language used by the team at Kansas State University (about whose solar thermochemical ammonia synthesis process I wrote last week), who saw quite clearly the advantages of a modular, scalable technology. You no longer need:
- Transportation infrastructure
- Politically stable region
- Technically advanced workforce
And, while that list is important, I’d add another item: ability to raise financing. As we’ve plainly seen in recent years, it is becoming infeasible to finance a $1+ billion greenfield ammonia plant: it simply costs too much. By eliminating some of those risks (distribution, politics, advanced training) and by reducing the scale of required capital, these projects may become significantly more viable.
Efficiencies of scale are meaningless if your technology is too big to build.
The Manthiram Lab only recently began its ammonia project, to develop “low-temperature, ambient pressure routes for the electrochemical synthesis of ammonia … in which water is used as the hydrogen source.” However, Manthiram and Schiller make it clear that there is more to solving this problem than favorable thermodynamics:
There are many challenges that remain with respect to the kinetics of these reactions that are certain to remain the focus of future research … For a wide range of reaction chemistries, efforts at the intersection of atomically and molecularly precise catalysts, in situ spectroscopy, and computational modeling of surfaces will have great impact on electrocatalyst discovery. Enzymatic catalysts, such as nitrogenases and carboxylases, may also either be incorporated into or provide inspiration for the design of electrocatalytic processes.
Schiffer and Manthiram, Electrification and Decarbonization of the Chemical Industry, September 2017
Nitrogenase-inspired catalysts were indeed the subject of a series of presentations at the conference, about which I will write in the future, but a different kind of electrochemical reaction was announced the next week by the team at Monash University, led by Doug MacFarlane.
Electro-synthesis of ammonia from nitrogen at ambient temperature and pressure in ionic liquids
While the team at MIT hasn’t yet published its results, the team at Monash is justifiably excited to do so: they have just reported the most “efficient” electrochemical ammonia synthesis process yet.
Several groups have investigated the reduction of N2 to NH3 from aqueous or alcohol-based solutions at room temperature at a variety of electrodes. However, only impractically low current-conversion efficiency (also known as faradaic efficiency, FE), below 7%, has been achieved …
The reason for the low faradaic efficiency of ammonia electro-synthesis is typically the reduction of water to hydrogen that occurs in the same region of potential. This is often dominant, in part because N2 is only sparingly soluble in most electrolytes. On the other hand, ionic liquids (ILs) can serve as excellent non-aqueous electrolytes and, as the water content in ILs can be much lower than in aqueous solutions, H2 evolution can be effectively suppressed. In addition, certain ILs are known to support high nitrogen solubility at room temperature, as much as 20 times higher than in water on a volumetric basis, making these particular ILs attractive as media for the nitrogen reduction process.
In this work, we demonstrate an electrochemical synthesis of ammonia with faradaic efficiency as high as 60% at ambient temperature and pressure. This high efficiency is achieved via the combination of a hydrophobic, high nitrogen-solubility IL electrolyte and a nanostructured Fe-based electrocatalyst.
MacFarlane et al, Electro-synthesis of Ammonia from Nitrogen at Ambient Temperature and Pressure in Ionic Liquids, November 2017
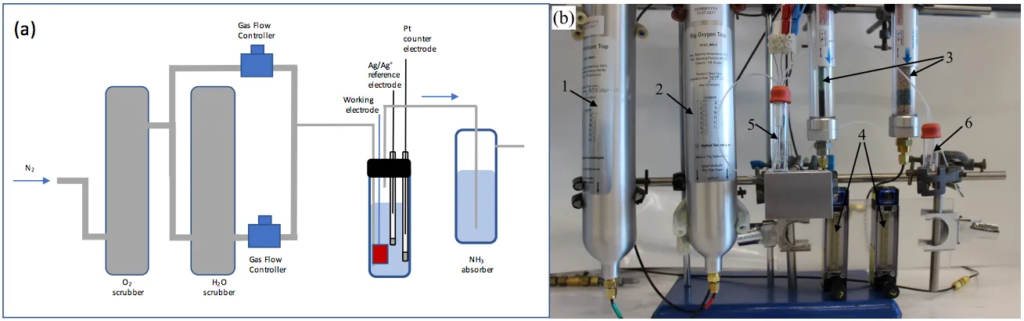
MacFarlane et al, Electro-synthesis of Ammonia from Nitrogen at Ambient Temperature and Pressure in Ionic Liquids, November 2017
While it has been aptly reported that this “electro-reduction technique could spearhead an ammonia economy,” and holds “exciting prospects for renewable energy,” the team at Monash still has significant work ahead.
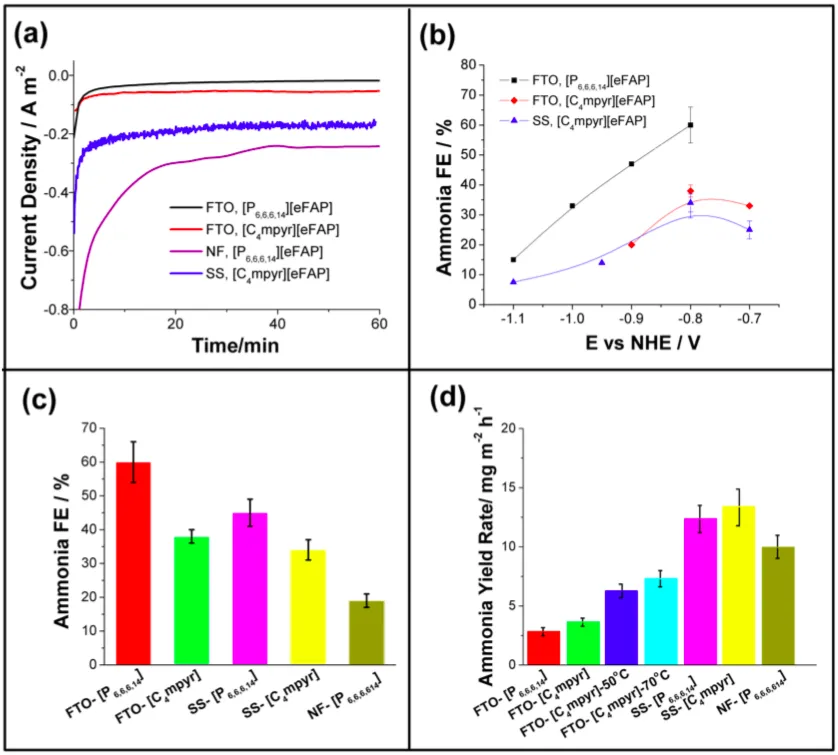
Faradaic efficiency is not the only measure nor a guarantee of success, and “the reduction currents obtained on this low surface area substrate are too small for practical application.”
However, by using different substrates to increase the working surface area, the researchers were able to increase the current density and the rate of ammonia production, but at the cost of faradaic efficiency.
But this points to the way forward for the researchers:
The decreased faradaic efficiencies on the more porous substrates suggests that N2 depletion, or build up of NH3, near the electrode during the reaction is limiting the rate of nitrogen reduction and shifting the balance in favour of proton reduction. Such mass transport limitations are common in electrochemical processes and can be suppressed in advanced cell design … It is important to note that the SS [stainless steel] cathodes used here are quite thin and flexible so that there is considerable scope to further increase yields by optimizing cathode thickness and also to implement, high surface area, thin-layer or spiral wound type electrode configurations …
The yields are currently un-optimized, and there is considerable scope for further development of the catalysts, along with optimization of other parameters such as electrode composition, structure and geometry.
MacFarlane et al, Electro-synthesis of Ammonia from Nitrogen at Ambient Temperature and Pressure in Ionic Liquids, November 2017
You can also read the full article at AmmoniaIndustry.com.