Future Ammonia Technologies: Electrochemical (part 2)
By Trevor Brown on December 27, 2017
Last week, in Part 1 of this series on electrochemical ammonia synthesis technologies, I quoted a recent article by researchers at MIT that identified avenues for future research and development. One option was a biomimicry approach, learning from “enzymatic catalysts, such as nitrogenases,” which can “either be incorporated into or provide inspiration for the design of electrocatalytic processes.”
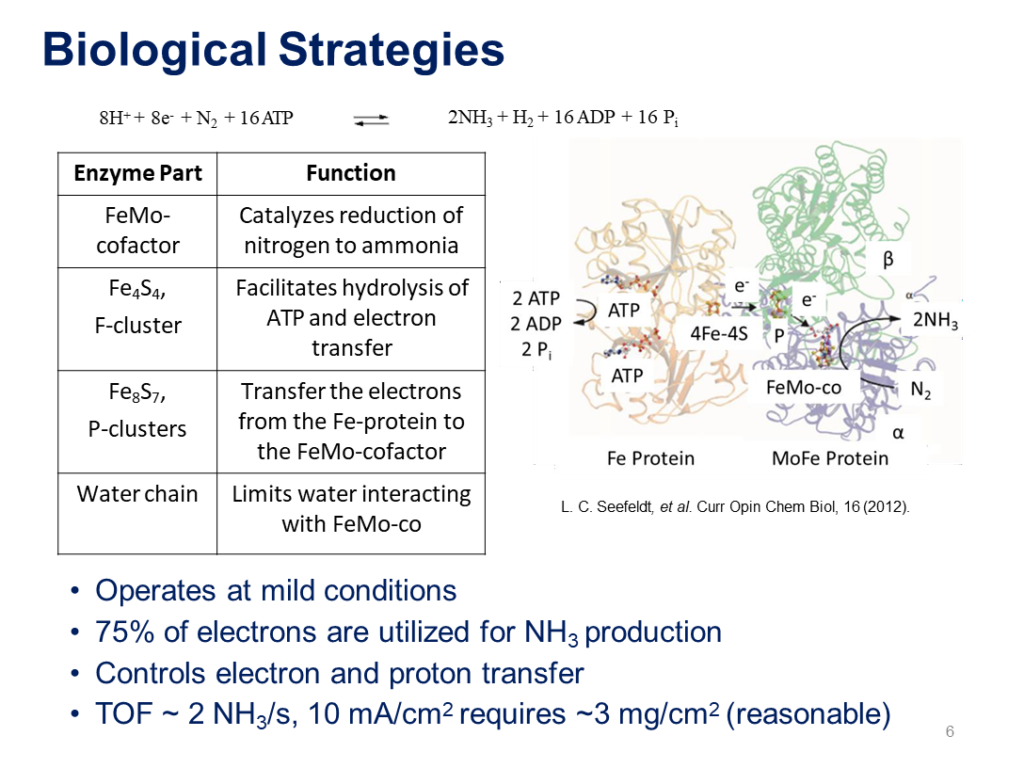
The nitrogenase enzyme, nature’s ammonia synthesis technology, was developed in an iterative innovation process, otherwise known as evolution, that took hundreds of millions of years to reach this level of efficiency. According to one group of electrochemists, who presented their results at the recent NH3 Energy+ conference, nitrogenase produces ammonia in nature with an enviable 75% process efficiency – so it’s no surprise that they are basing their industrial technology on it.
Nitrogenase Inspired Peptide-Functionalized Catalyst for Efficient, Emission-Free Ammonia Production
Proton OnSite, a global leader in hydrogen generation using PEM electrolysis technology, has been investigating renewable ammonia production for some years, looking at two pathways: one, electrolytic hydrogen as a feedstock for Haber-Bosch and, two, direct electrochemical synthesis.
Earlier this year, Proton OnSite became the US subsidiary of Nel Hydrogen. Like Yara, Nel used to be part of Norsk Hydro and thus has a long history of producing renewable ammonia at Rjukan and Glomfjord in Norway, where hydroelectricity powered the largest electrolyzers in the world (135 MW each), from the 1920s to the 1990s.
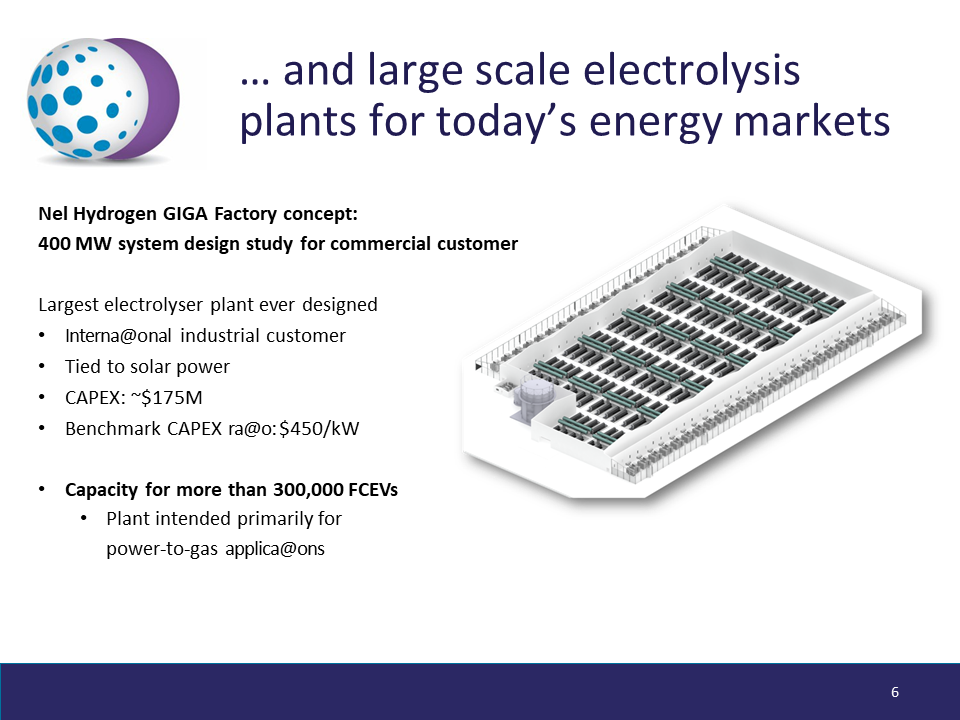
Now, Nel is the company at the forefront of the electrolyzer capex cost-curve, and the reason the International Energy Agency (IEA) considers renewable ammonia to be cost-competitive with fossil ammonia in some regions already.
This year’s key figure was $450/kW. This was the new-low capex ratio for Nel’s GIGA Factory: a $175 million, 400 MW electrolytic hydrogen production plant, designed for integration with solar power in the industrial power-to-gas market. This capex ratio is roughly half the previously announced figures, and represents a major step down the electrolyzer cost-curve.
According to last month’s NH3 Energy+ conference presentation by Proton Onsite’s Steve Szymanski, electrolysis technology is “reaching relevant scale for ‘green ammonia,’” and therefore this renewable hydrogen production pathway “will lead any transition” away from fossil ammonia.
However, the direct electrochemical ammonia pathway is the “long term option,” which is why Proton has been leading a “groundbreaking research project to enhance the efficiency and selectivity of an electrochemical ammonia synthesis process.”
Electrochemical solutions are well-suited to modularity and integration with renewable energy sources and can operate at much milder temperatures and pressures, but a catalyst is needed which is selective to ammonia generation vs competing reactions.
To address the challenge above, Proton OnSite, in collaboration with the University of Arkansas and Case Western Reserve University have demonstrated feasibility for improved ammonia selectivity through tailoring nanoparticle catalyst morphology and using peptides derived from nitrogenase (a nitrogen-splitting enzyme in nature) to direct the desired reaction. Proton’s expertise in electrode fabrication, cell design and water management, combined with the universities’ expertise in catalyst design and synthesis, were combined to fabricate single cell stacks and demonstrate ammonia production from nitrogen over argon controls. The main goal of the next phase is to further enhance the selectivity based on the directions determined in Phase I, and develop an appropriate cell configuration for the resulting electrode.
… Processes will also be developed for manufacture of the catalyst system at larger scale, for integration with Proton’s existing fabrication capability at scale.
Szymanski et al, Nitrogenase Inspired Peptide-Functionalized Catalyst for Efficient, Emission-Free Ammonia Production, November 2017
Szymanski was not the only one discussing this project: his partners at Case Western Reserve and the University of Arkansas both gave presentations at the NH3 Energy+ conference.
Exploring Peptide-Bound Catalysts for Electrochemical Ammonia Generation
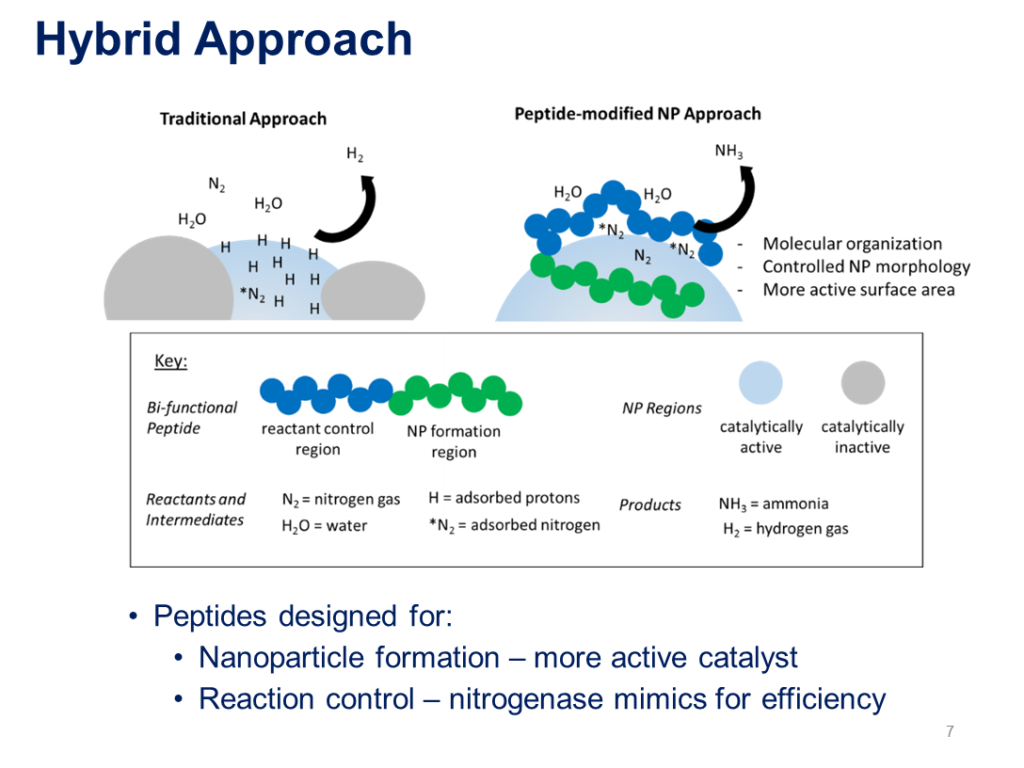
The team at Case Western Reserve University reported their work developing “manufacturable, peptide-bound electrocatalysts inspired by the enzyme nitrogenase,” and testing these for ammonia production in Proton’s anion-exchange membrane (AEM) system.
Distinct from the PEM that Proton uses for hydrogen production, the AEM “is ideal for ammonia synthesis because the alkaline configuration allows the utilization of a wider array of low-cost catalysts.”
While electrochemical ammonia generation is promising, the low-temperature and low-pressure testing to date has shown low efficiencies (<1%), highlighting the need for creative catalyst approaches … Meanwhile, the enzyme nitrogenase which reduces nitrogen in nature, operates at mild temperatures and pressures with high efficiency (66%) … In this work, peptide-functionalized catalysts are developed, characterized and tested in and AEM-based system. The novel materials show promise compared to conventional catalyst approaches.
Loney et al, Exploring Peptide-Bound Catalysts for Electrochemical Ammonia Generation, November 2017
Design of Iron-Nickel Nanocatalysts for Low-Temperature Electrochemical Ammonia Generation
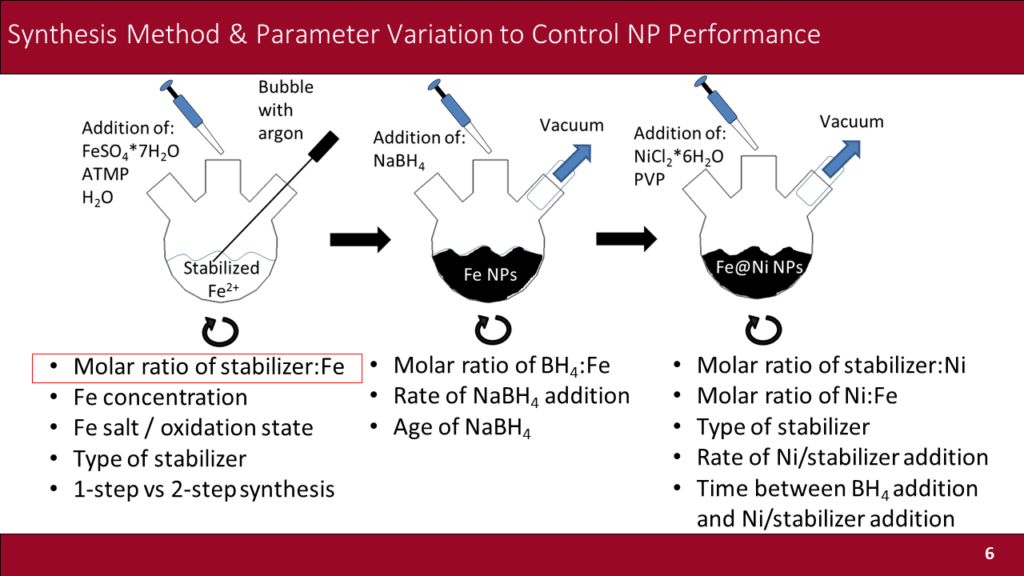
Lauren Greenlee, from the University of Arkansas, also reported on their work addressing the selectivity challenge by designing nano-scale catalysts.
This part of the project was supported by funding from the US Department of Energy; I wrote about the DOE’s sustainable ammonia synthesis funding back in January 2017.
We aim to develop nanoparticulate catalysts to address [the] selectivity issue for electrochemical nitrogen reduction. We synthesize non-precious metal-based catalysts comprised of iron and nickel, which, as a bimetallic material, are theoretically-predicted to potentially result in optimal surfaces for nitrogen reduction … we also design our nanocatalysts such that the local surface environment of the catalyst is controlled. To control the local surface environment, we use specifically-structured short-chain peptide sequences inspired from the structure of the enzyme nitrogenase, which is a bacterial enzyme that naturally reduces nitrogen to ammonia. In particular, we study how peptide sequence affects water and nitrogen transport to the catalyst surface.
Greenlee et al, Design of Iron-Nickel Nanocatalysts for Low-Temperature Electrochemical Ammonia Generation, November 2017
Of course, while significant, this particular project is by no means the only one underway in the US today: in Part 3 of this series of articles, I’ll introduce six completely different electrochemical ammonia synthesis technologies currently under development with funding from the DOE’s ARPA-E.
You can also read the full article at AmmoniaIndustry.com.