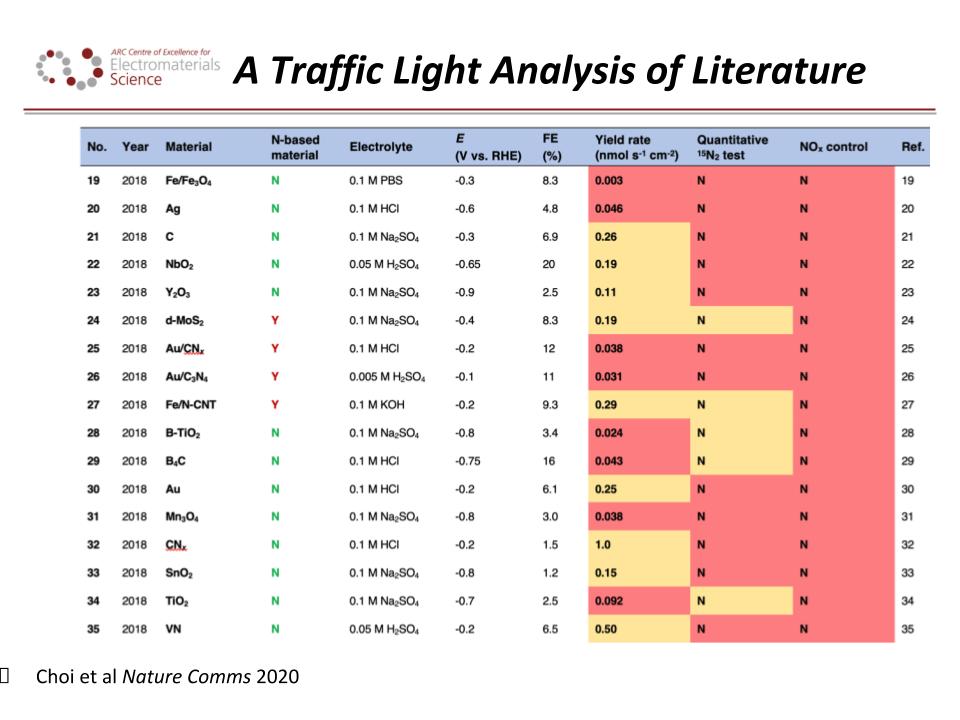
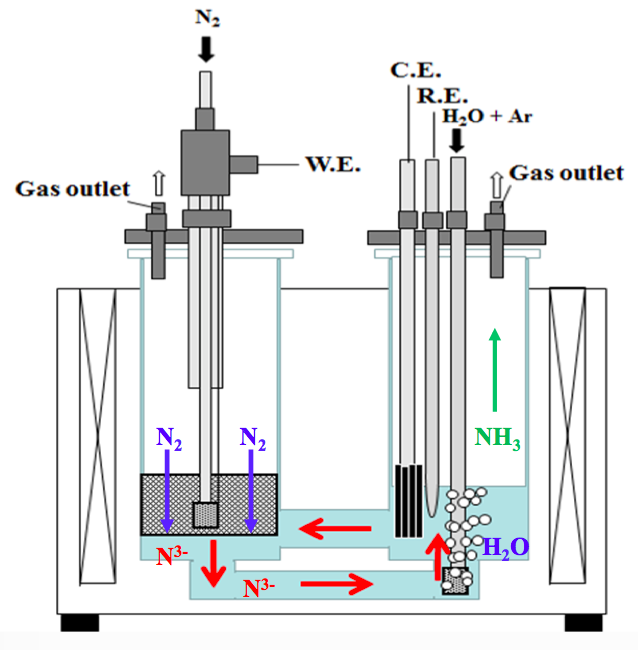
Content Related to Aristotle University
Presentation
Alternatives to Ammonia Synthesis: An Electrochemical Haber-Bosch Process
Several alternatives to the existing process for ammonia synthesis, the Haber-Bosch Process, have been proposed in the past two decades, including the electrochemical synthesis in aqueous, molten salt or solid electrolyte cells. The present work reviews results of recent efforts (last 3 years) for the electrochemical synthesis of ammonia. An Electrochemical Haber-Bosch Process is also demonstrated. The proposed BaZrO3 – based protonic ceramic membrane reactor combines hydrogen production via the reactions of methane steam reforming and water-gas shift at the anode (Ni-composite) with ammonia synthesis from N 2 and protons (H + ) at the cathode (VN-Fe). Hydrogen extraction from…
Article
Panel discussion on next-generation ammonia synthesis
Kevin Rouwenhorst December 03, 2020
This year’s Ammonia Energy Conference included a panel discussion on next-generation ammonia synthesis, moderated by Sarb Giddey (CSIRO, Australia), and featuring panelists Doug MacFarlane (Monash University, Australia), Karthish Manthiram (MIT, United States), and Michael Stoukides (Aristotle University of Thessaloniki, Greece). The panel discussed the direct fixation of nitrogen in the form of ammonia from water and air in a single electrochemical device, which is considered the “holy grail” of ammonia synthesis. During the panel, the participants gave their perspectives on the state of the art, and the obstacles and opportunities for progress.
Article
Electrochemical ammonia synthesis in South Korea
Trevor Brown June 09, 2017
One of the many encouraging announcements at the recent Power-to-Ammonia conference in Rotterdam was the news that the Korea Institute of Energy Research (KIER) has extended funding for its electrochemical ammonia synthesis research program by another three years, pushing the project forward through 2019. KIER's research target for 2019 is significant: to demonstrate an ammonia production rate of 1x10-7 mol/s·cm2. If the KIER team can hit this target, not only would it be ten thousand times better than their 2012 results but, according to the numbers I'll provide below, it would be the closest an electrochemical ammonia synthesis technology has come to being commercially competitive.
Presentation
Progress in the Electrochemical Synthesis of Ammonia
Ammonia is one of the most important and widely produced chemicals worldwide with a key role in the growth of human population. Nowadays, the main route for ammonia synthesis is the Haber-Bosch process, developed one century ago. In this process, Fe-based catalysts are usually employed at temperatures between 400 and 500°C and pressures between 130 and 170 bar. As opposed to the industrial process, in nature, plants and bacteria have been producing ammonia for millions of years at mild conditions. Atmospheric nitrogen is reduced by solvated protons on the FeMo cofactor of the metalloenzyme nitrogenase. The natural method of nitrogen…