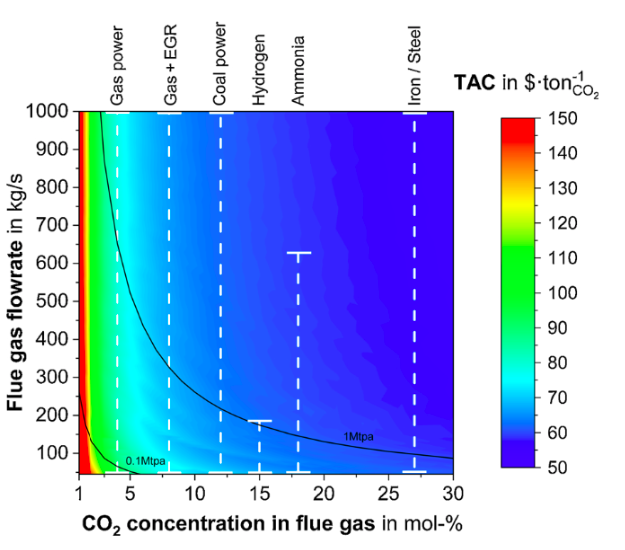
In discussions of carbon capture technology for low-carbon ammonia production, there are two informal rule-of-thumb numbers: 60% and 90%. We know we can capture, at very little additional cost, over 60% of the CO2 from a natural gas-based ammonia plant because this is the process gas (the byproduct of hydrogen production). Many ammonia plants already utilize this pure CO2 stream to produce urea or to sell as food grade CO2. The remaining CO2 emissions are in the much more dilute flue gas (the product of fuel combustion to power the process). For some decades we have assumed we could capture most of this but the lingering question has always been: how much of that flue gas is economically feasible to capture? A team of researchers at Imperial College London has just published a fascinating study into this question, entitled “Beyond 90% capture: Possible, but at what cost?” The paper quantifies the tipping point — ranging from 90% to 99%, depending on flow rates and concentration — beyond which it is easier to capture CO2 directly from the air than it is to capture more flue gas emissions.
Content Related to Imperial College London
Article
A rigorous protocol for measuring electrochemical ammonia synthesis rates
Trevor Brown May 26, 2019
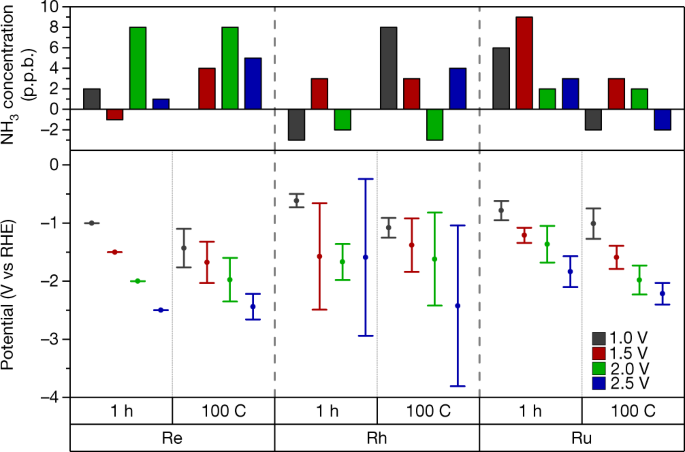
NEWS BRIEF: A paper published this week in Nature addresses the challenge of accurately reporting synthesis rates for electrochemical ammonia production technologies. According to the authors, from Stanford University, the Technical University of Denmark (DTU), and Imperial College London, it is not always clear if new technologies really synthesize ammonia, or if the researchers simply measured contaminants. This is because, at experimental scale, materially significant amounts of ammonia (or other nitrogen-containing molecules) could be present in the air, membranes, catalysts, or simply the researchers' breath. To support the development of viable electrochemical ammonia synthesis technologies, the authors propose "benchmarking protocols," and "a standardized set of control experiments."