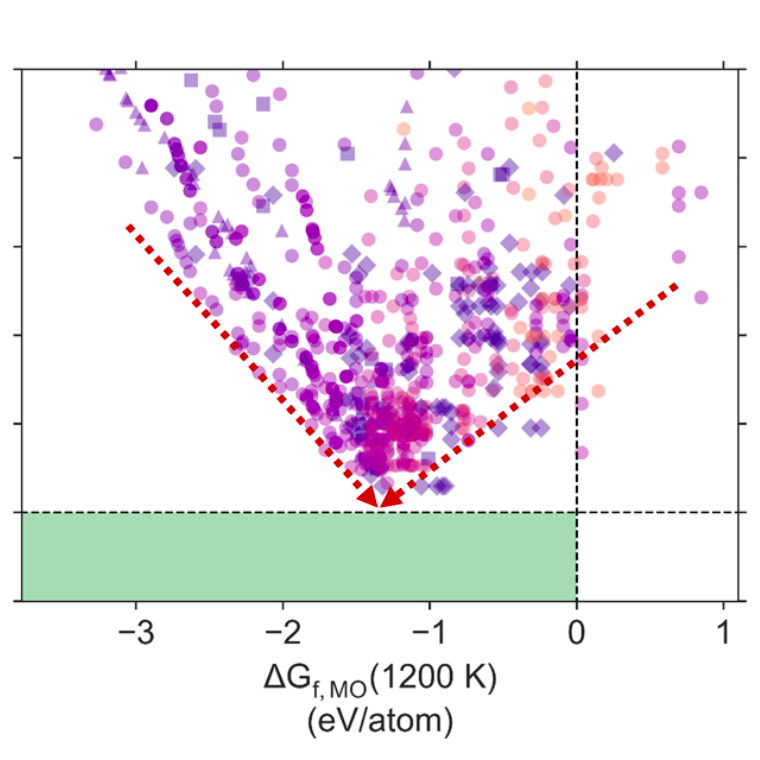
This paper presents an innovative approach of producing energy-dense, carbon-neutral liquid ammonia as a means of energy carrier. The approach synergistically integrates microwave reaction chemistry with novel heterogeneous catalysis that decouples N2 activation from high temperature and high pressure reaction, altering reaction pathways and lowering activation energy. Results have shown that ammonia synthesis can be carried out at 280 ℃ and ambient pressure to achieve ~1 mmol NH3/g cat. /hour over supported Ru catalyst systems. Adding promoters of K, Ce and Ba has significantly improved the ammonia production rate over Ru-based catalysts that could be attributing to enhanced electromagnetic sensitivity…
Content Related to National Energy Technology Laboratory (NETL)
Presentation
Microwave Catalysis for Ammonia Synthesis Under Mild Reaction Conditions
Jianli HuHanjing TianYan LuoXinwei BaiDushyant ShekhawatChristina WildfireVictor AbdelsayedMichael J. SpencerRobert A. DagleStephen DavidsonAlbert E. Stiegman
A scalable, cost-effective catalytic process of ammonia synthesis is developed by using microwave excitation under mild reaction conditions. In this research project funded by DOE ARPA-E, our interdisciplinary team of WVU, NETL, PNNL, FSU and two industrial partners have demonstrated that ammonia synthesis can be carried out at 200-300 °C and ambient pressure. This transformational process integrates system elements of electromagnetic sensitive catalysts and microwave reactor design. Taking advantages of state-of-the art non-equilibrium microwave plasma technology, catalytic ammonia synthesis undergoes a new reaction pathway where the barrier for the initial dissociation of the dinitrogen is decoupled from the bonding energy…
Article
Future Ammonia Technologies: Plasma, Membrane, Redox
Trevor Brown December 08, 2017
I wrote recently about two pathways for ammonia production technology development: improvements on Haber-Bosch, or electrochemical synthesis. Last week, I covered some of these Haber-Bosch improvements; next week, I'll write about electrochemical processes. This week, I want to write about some innovations that don't fit this two-way categorization: they don't use electrochemistry and they don't build upon the Haber-Bosch process, and that might be the only thing that links them.