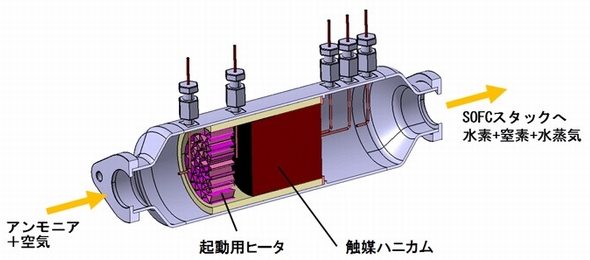
Hydrogen is the primary fuel source for fuel cells. However, the low volume density and difficulty in storage and transportation are major obstacles for the practical utilization. Among various hydrogen carriers, ammonia is one of the promising candidates because of its high hydrogen density and boiling point and ease in liquefaction and transportation. The reaction temperature of ammonia cracking to nitrogen and hydrogen, being about 600°C or higher, is close to the operating temperature of solid oxide fuel cells (SOFCs). The integration of these two devices is beneficial in terms of heat and energy managements and will lead to the…
Content Related to Nippon Shokubai
Presentation
Development of Materials and Systems for Ammonia-Fueled Solid Oxide Fuel Cells
Koichi EguchiYosuke TakahashiHayahide YamasakiHidehito KuboAkihiro OkabeTakenori IsomuraTakahiro Matsuo
Hydrogen is the primary fuel source for fuel cells. However, the low volume density and difficulty in storage and transportation are major obstacles for the practical utilization. On-site generation of hydrogen from its carrier is an effective method for the fuel supply. Among various hydrogen carriers, ammonia is one of the promising candidates. Ammonia has high hydrogen density. The boiling point of ammonia is relatively high, leading to the ease in liquefaction and transportation. Hydrogen can be produced from ammonia with a mildly endothermic process. The reaction temperature of ammonia cracking is about 600˚C or higher which is close to…
Article
Green Ammonia Consortium: Bright Prospects in Japan for Ammonia as an Energy Carrier
Stephen H. Crolius October 05, 2017
In the last 12 months ... In July 2017, 19 companies and three research institutions came together to form the Green Ammonia Consortium. Before this development, it was unclear whether ammonia would find a significant role in Japan’s hydrogen economy. In the wake of this announcement, however, ammonia seems to have claimed the leading position in the race among potential energy carriers.
Article
Ammonia-Fueled Solid Oxide Fuel Cell Advance at Kyoto University
Stephen H. Crolius July 20, 2017
Earlier this month the Eguchi Laboratory at Kyoto University announced advances in ammonia-fueled solid oxide fuel cell technology. The lab was able to produce a functioning fuel cell with a power output of one kilowatt. The device attained “direct current power generation efficiency” in excess of 50% and reached 1,000 hours of continuous operation.
Presentation
Research and Development of Ammonia-fueled SOFC Systems
Koichi EguchiAtthapon SrifaTakeou OkanishiHiroki MuroyamaToshiaki MatsuiMasashi KishimotoMotohiro SaitoHiroshi IwaiHideo YoshidaMasaki SaitoTakeshi KoideHiroyuki IwaiShinsuke SuzukiYosuke TakahashiToshitaka HoriuchiHayahide YamasakiShohei MatsumotoShuji YumotoHidehito KuboJun KawaharaAkihiro OkabeYuki KikkawaTakenori Isomura
Ammonia is a promising hydrogen carrier because of its high hydrogen density, low production cost, and ease in liquefaction and transport. Ammonia decomposes into nitrogen and hydrogen through a mildly endothermic process. The ammonia decomposition temperature is close to the operating conditions of solid oxide fuel cells (SOFCs). Therefore, the integration of these two devices is beneficial in terms of efficient heat and energy managements and will lead to the development of simplified generation systems. We have investigated three types of ammonia-fueled SOFC systems. In one system, ammonia is directly supplied to the anode chamber. Ammonia decomposes into nitrogen and…
Presentation
Current progress in development of NH3-fueled solid-state fuel cell systems
Takeou OkanishiKaname OkuraJun YangHiroki MuroyamaToshiaki MatsuiMasashi KishimotoMotohiro SaitoHiroshi IwaiHideo YoshidaKoichi EguchiHiroyuki IwaiK. InaokaShinsuke SuzukiYosuke TakahashiToshitaka HoriuchiHayahide YamasakiHideyuki MatsumotoHidehito KuboJun KawaharaAkihiro OkabeYuki KikkawaT. NegishiS. Watanabe
Current progress in development of NH3-fueled solid-state fuel cell systems T. Okanishi*, K. Okura, J. Yang, H. Muroyama, T. Matsui, M. Kishimoto, M. Saito, H. Iwai, H. Yoshida, K. Eguchi, Kyoto University; H. Iwai, K. Inaoka, S. Suzuki, Y. Takahashi, Noritake; T. Horiuchi, H. Yamasaki, Nippon Shokubai; S. Matsumoto, H. Kubo, Toyota Industries; J. Kawahara, A. Okabe, Mitsui Chemical; Y. Kikkawa, T. Negishi, S. Watanabe, Tokuyama