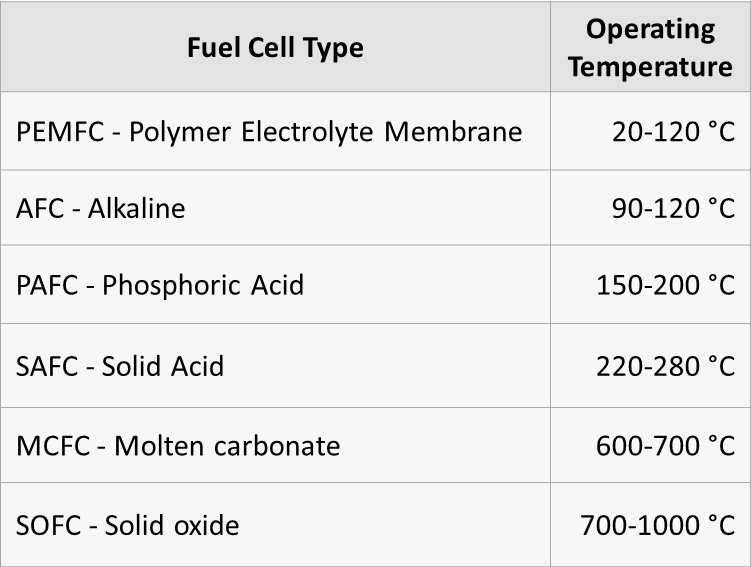
Liquid ammonia can be used as an alternative hydrogen carrier and can be decomposed over catalysts to create a high purity hydrogen stream for fuel cell applications. Ammonia decomposition is typically catalyzed using supported ruthenium catalysts. Current ruthenium catalysts are expensive and often require reaction temperatures of 650 °C to attain complete conversion [1]. For the hydrogen produced from ammonia decomposition to be efficiently used in proton exchange membrane fuel cells, operating temperatures need to be considerably lowered and effluent concentrations of ammonia need to be minimized to avoid poisoning of the membrane [2]. Therefore, it is of interest to…
Content Related to University of South Carolina
Presentation
Material Discovery and High Throughput Exploration of Ru Based Catalysts for Low Temperature Ammonia Decomposition
High throughput experimentation gives us the unique ability to generate massive, multidimensional datasets that are not typical for heterogeneous catalysis studies. Here, we show the synthesis and catalytic screening of over 100 different Ru based bimetallic catalyst combinations using 33 different metals that were synthesized via incipient wetness impregnation. The catalysts were analyzed using Wide Angle X-ray Scattering (WAXS) for phase identification. Catalysts were screened for ammonia decomposition activity using a 16-channel parallel plug flow reactor. Fourier transform infrared (FT-IR) imaging was used to analyze all 16 effluent streams in parallel in under one minute. All results obtained from WAXS…
Article
REFUEL Ammonia Use-Side Funding Awards
Stephen H. Crolius December 23, 2016
Six of the projects designated for funding by the ARPA-E REFUEL announcement on December 15 involve technologies on the use side of the ammonia energy space. Three focus on generating hydrogen from ammonia. Two focus on fuel cells that convert ammonia to electricity. One project involves both ammonia synthesis and use.