Ammonia Synthesis
Importance of Reaction Mechanism Involved in Design of the Catalyst and the Reactor for Future Ammonia Synthesis
Yara and BASF open their brand-new, world-scale plant, producing low-carbon ammonia
The newest ammonia plant on the planet has opened in Freeport, Texas. A joint venture between Yara and BASF, this world-scale ammonia plant uses no fossil fuel feedstock. Instead, it will produce 750,000 metric tons of ammonia per year using hydrogen and nitrogen delivered directly by pipeline. The plant's hydrogen contract is structured so that the primary supply is byproduct hydrogen, rather than hydrogen produced from fossil fuels, and therefore the Freeport plant can claim that its ammonia has a significantly reduced carbon footprint. This new ammonia plant demonstrates three truths. First, low-carbon merchant ammonia is available for purchase in industrial quantities today: this is not just technically feasible but also economically competitive. Second, carbon intensity is measured in shades of grey, not black and white. Ammonia is not necessarily carbon-free or carbon-full, but it has a carbon intensity that can quantified and, in a carbon-constrained economy, less carbon content equates to higher premium pricing. Third, the ammonia industry must improve its carbon footprinting before it can hope to be rewarded for producing green ammonia.
Joyn Bio: microbial engineering for sustainable nitrogen
Six months ago, in September 2017, I reported a $100 million joint venture announcement between Bayer and Ginkgo Bioworks that aimed to engineer nitrogen-fixing microbes, which could be put into seed coatings and provide nutrients to non-legume crops. Now, the joint venture has been named, and Joyn Bio is staffing up. For the ammonia industry, this represents potential demand destruction at a significant scale in the coming decades.
Improvement of Haber-Bosch: Adsorption vs. Absorption
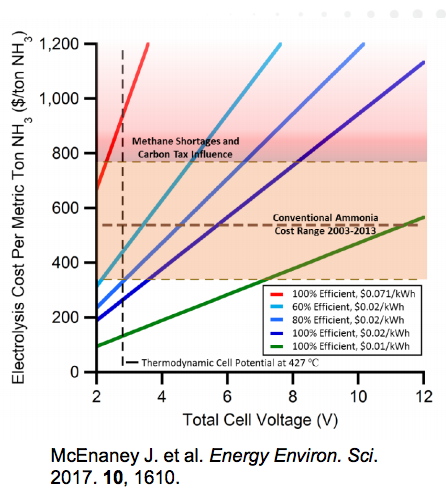
At the recent NH3 Energy+ Topical Conference, Grigorii Soloveichik described the future of ammonia synthesis technologies as a two-way choice: Improvement of Haber-Bosch or Electrochemical Synthesis. Two such Haber-Bosch improvement projects, which received ARPA-E-funding under Soloveichik's program direction, also presented papers at the conference. They each take different approaches to the same problem: how to adapt the high-pressure, high-temperature, constant-state Haber-Bosch process to small-scale, intermittent renewable power inputs. One uses adsorption, the other uses absorption, but both remove ammonia from the synthesis loop, avoiding one of Haber-Bosch's major limiting factors: separation of the product ammonia.
The Future of Ammonia: Improvement of Haber-Bosch ... or Electrochemical Synthesis?
During our NH3 Energy+ Topical Conference, hosted within AIChE's Annual Meeting earlier this month, an entire day of presentations was devoted to new technologies to make industrial ammonia production more sustainable. One speaker perfectly articulated the broad investment drivers, technology trends, and recent R&D achievements in this area: the US Department of Energy's ARPA-E Program Director, Grigorii Soloveichik, who posed this question regarding the future of ammonia production: "Improvement of Haber-Bosch Process or Electrochemical Synthesis?"
254th ACS Meeting, Energy and Fuels Symposium “The Ammonia Economy” — Synthesis, Utilization & Nitrogen Reduction
In late August, the day before the exciting solar eclipse, the Ammonia Economy symposium was held as part of the Energy and Fuels Division of the American Chemical Society (ACS) National Meeting in Washington DC. This marks the third gathering of Ammonia related research since 2015 at the national level ACS conference. This year, in addition to the important focus on chemistries for the utilization of ammonia, the rapidly developing field of homogeneous catalysts and biological processes for nitrogen fixation was included as a major theme.
NH3 Fuel Association announces New Sponsor; Evening Reception at AIChE Annual Meeting on November 1st
The NH3 Fuel Association has finalized details of its Sponsors Reception on Wednesday November 1 at the AIChE Annual Meeting in Minneapolis, and has also announced an additional sponsor for the conference: Starfire Energy.
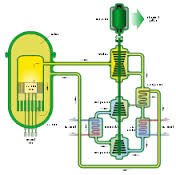
Terrestrial Energy and the Production of Carbon-Free Ammonia
On January 24, the nuclear energy company Terrestrial Energy USA informed the United States Nuclear Regulatory Commission of its plans “to license a small modular, advanced nuclear reactor in the United States.” Many steps later – sometime in the 2020s – the American subsidiary of the Canadian company Terrestrial Energy, Inc., hopes to bring its IMSR technology to market. IMSR stands for integral molten salt reactor. The IMSR stands apart from conventional nuclear technology on several dimensions. On the dimension of operating temperature, the IMSR is hot enough that it can be beneficially integrated with high-temperature industrial processes. According to the company’s research, ammonia production could be a candidate for such integration.
Methane to Ammonia via Pyrolysis
Eric McFarland, Professor of Chemical Engineering at the University of California Santa Barbara, likes fossil fuels and nuclear energy and is unimpressed by the menu of renewable energy technologies. But he is worried about climate change and he has an original view on how to modify our current energy system so that we don’t overload the atmosphere with CO2. He believes the key will be to separate fossil hydrocarbons into gaseous hydrogen and solid carbon. The chemistry he is developing in this area involves transferring “electrochemical potential” from hydrocarbons to alternative energy carriers. Ammonia is an energy carrier that McFarland believes is especially promising.