Cracking Ammonia
Next Generation Technology Integration Platform for Low- and Zero-Carbon Ammonia Production and Utilization
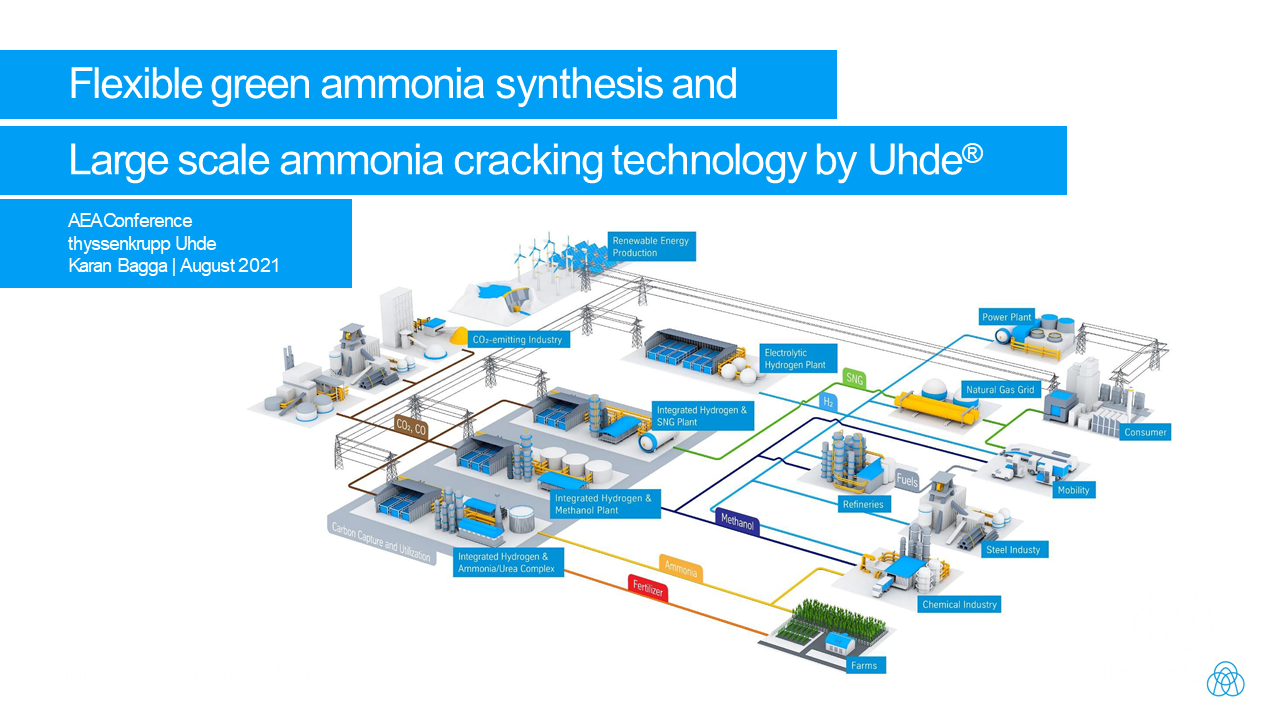
thyssenkrupp to expand electrolyser production five-fold
thyssenkrupp's annual production output of 1 GW alkaline water electrolysis cells will expand five-fold, thanks to new funding from Germany's Federal Ministry of Education and Research. There is also federal funding for two additional projects: H2Mare (offshore ammonia production), and TransHyDE (ammonia cracking solutions).
New materials for cracking catalysts
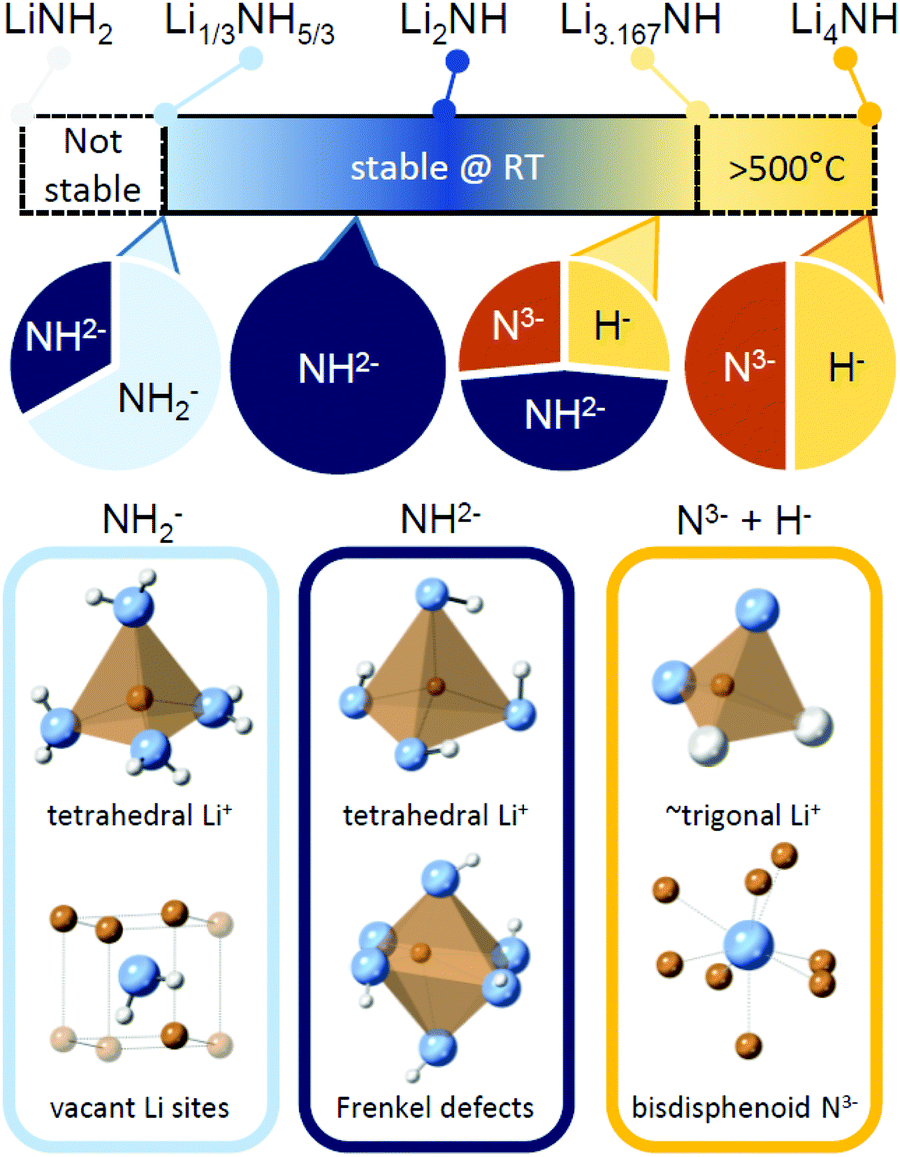
Among the many challenges for cracking researchers is their choice of material to build their catalysts from. There is hope that cheaper, more readily-available materials will replace the Ruthenium-based catalysts that have dominated the field up to this point. This week two new pieces of research suggest a way forwards using alkali metal-based materials: Lithium and Calcium.
Production and utilization of green ammonia: KIER’s current status and future plans
Doosan Heavy Industries, POSCO and RIST to develop ammonia gas turbines
Two members of the newly-launched Korean Green Ammonia Alliance - Doosan and POSCO - signed an MoU this week with the Pohang-based Research Institute of Industrial Science & Technology (RIST) to develop "Clean Ammonia-Fueled Gas Turbines".
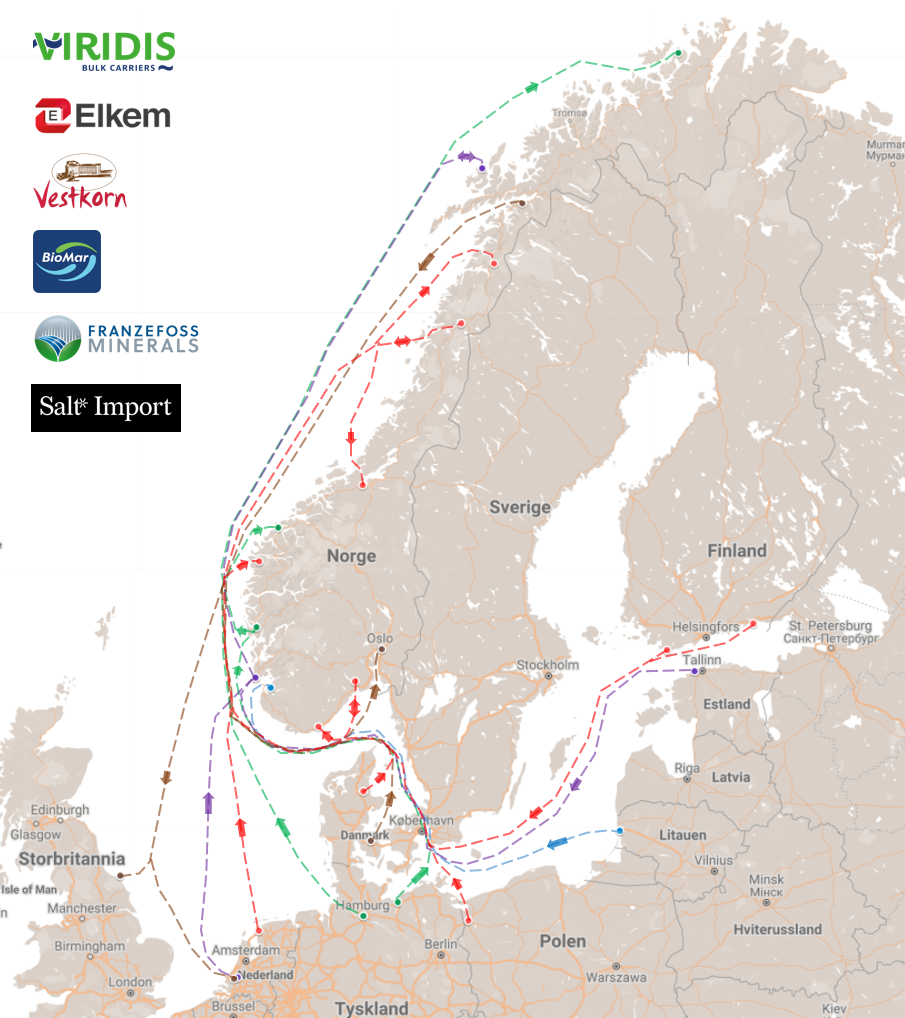
The Ammonia Wrap: an ammonia-powered shipping network in northern Europe and more
This week: an ammonia-powered shipping network in northern Europe, Sluiskil update, green projects in Uruguay, green ammonia in Ireland: a new update!, Euronav to develop ammonia-powered tankers, ammonia part of Equinor's net-zero by 2050 strategy, H2Site to install first on-site crackers in France and updates from Australia.
The Ammonia Wrap: no major obstacles for NoGAPS success and more
Welcome to the Ammonia Wrap: a summary of all the latest announcements, news items and publications about ammonia energy. This week: latest report from NoGAPS, Viking Energy project takes another step, more collaborations for Yara, thyssenkrupp to invest in cracking R&D, investment in clean hydrogen technology in the USA, world-first visualisation of ammonia combustion in a spark-ignition engine and our numbers of the week.
The Ammonia Academic Wrap: "seamless" cracking, improving Haber Bosch, a novel green power-to-ammonia-to-power solution and a review into the use of ammonia as a fuel
Welcome to the Ammonia Academic Wrap: a summary of all the latest papers, developments and emerging trends in the world of ammonia energy R&D. This week: "seamless" ammonia cracking tech from Northwestern, a new electrolysis catalyst, successful integration of ammonia synthesis and separation for improved efficiency, more research needed into transition metal catalysts for Haber Bosch, a novel, green power-to-ammonia to power system and a review on ammonia as a potential fuel.