Electrochemical Ammonia
Energy Storage through Electrochemical Ammonia Synthesis Using Proton-Conducting Ceramics
Technoeconomic Requirements for Sustainable Ammonia Production
A Techno-Economic Model for Renewable Ammonia By Electrochemical Synthesis with Proton Conductive Membrane
Synthesis of Ammonia By RF Non-Thermal Plasma over Ni-MOF-74
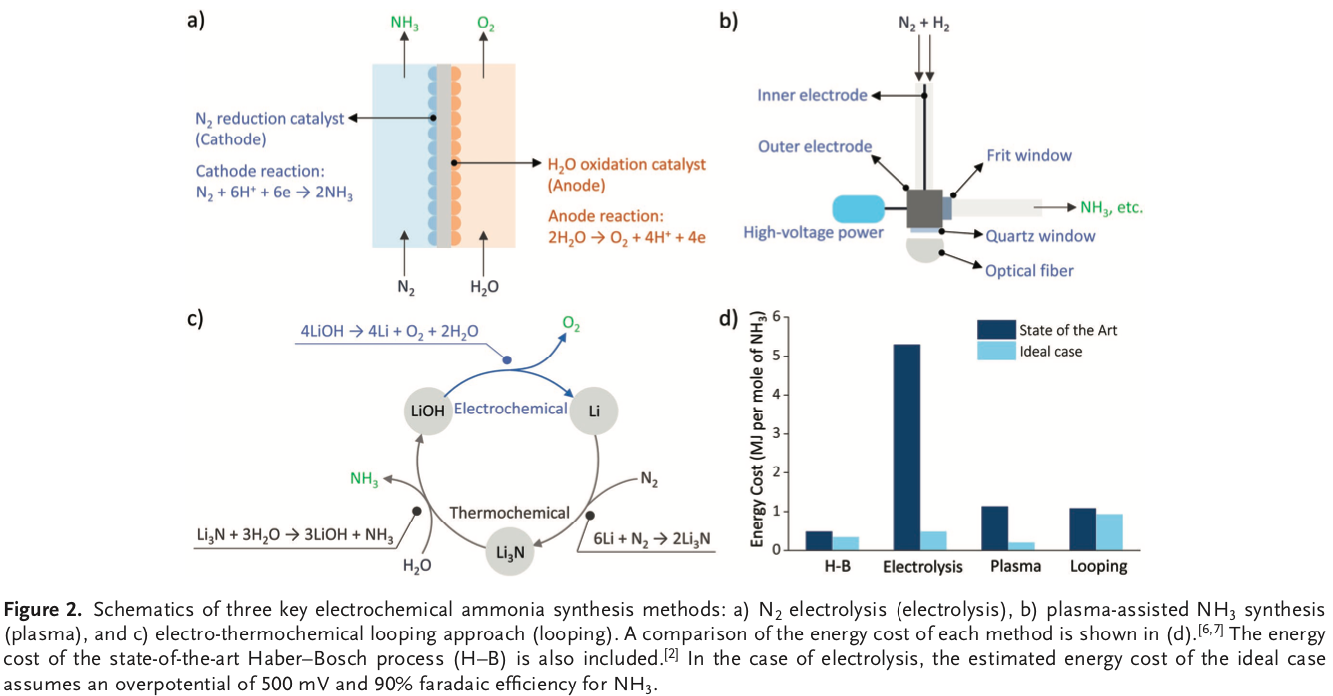
The global quest to decarbonize ammonia production
NEWS BRIEF: The industrial process for ammonia production is increasingly being recognized as a target for decarbonization - by researchers, investors, regulators, and the producers themselves. Demonstrating this shift in awareness, Chemical and Engineering News (C&EN), one of the flagship publications of the American Chemical Society (ACS), this week published an in-depth review of global research and development efforts and demonstration plants for sustainable ammonia synthesis. Its review is all-encompassing, from near-term feasible renewable Haber-Bosch plants, to long-term research areas of electrochemistry, photocatalysis, and bioengineering.
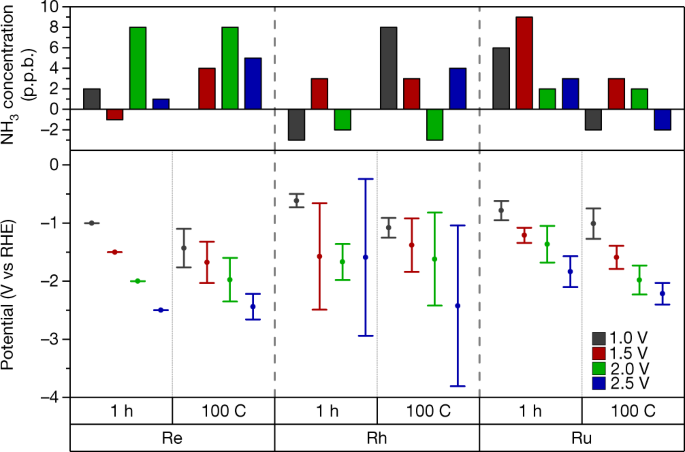
A rigorous protocol for measuring electrochemical ammonia synthesis rates
NEWS BRIEF: A paper published this week in Nature addresses the challenge of accurately reporting synthesis rates for electrochemical ammonia production technologies. According to the authors, from Stanford University, the Technical University of Denmark (DTU), and Imperial College London, it is not always clear if new technologies really synthesize ammonia, or if the researchers simply measured contaminants. This is because, at experimental scale, materially significant amounts of ammonia (or other nitrogen-containing molecules) could be present in the air, membranes, catalysts, or simply the researchers' breath. To support the development of viable electrochemical ammonia synthesis technologies, the authors propose "benchmarking protocols," and "a standardized set of control experiments."
Literature review: Electrochemical Ammonia Synthesis and Ammonia Fuel Cells
The journal Advanced Materials recently published an article that reviews electrochemical ammonia technologies for both synthesis and power generation. In addition to presenting a range of technologies under development, the authors, based at the University of Delaware, present "perspectives in the technical challenges and possible remedies."