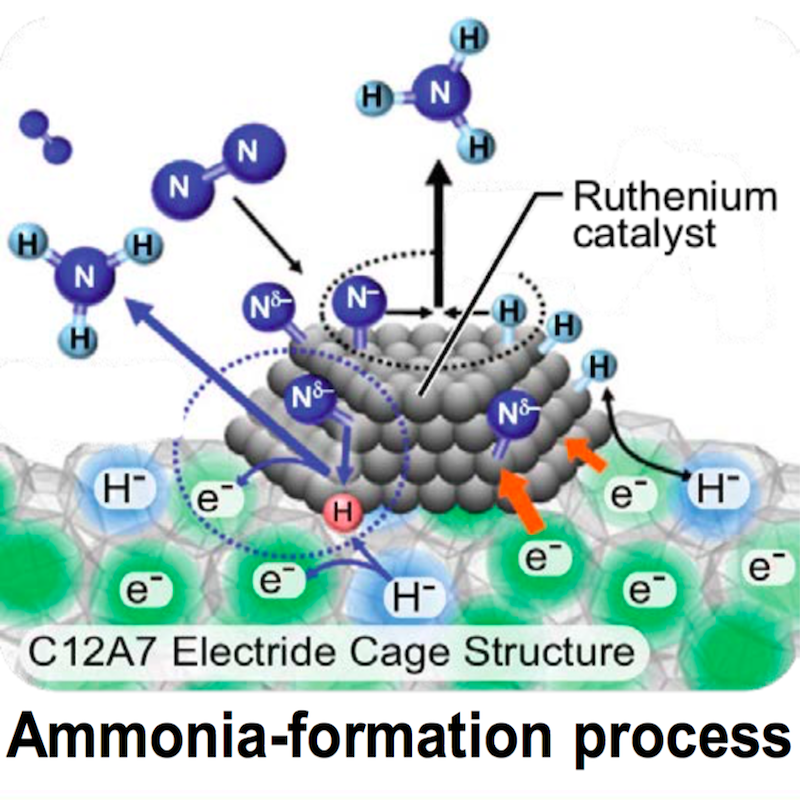
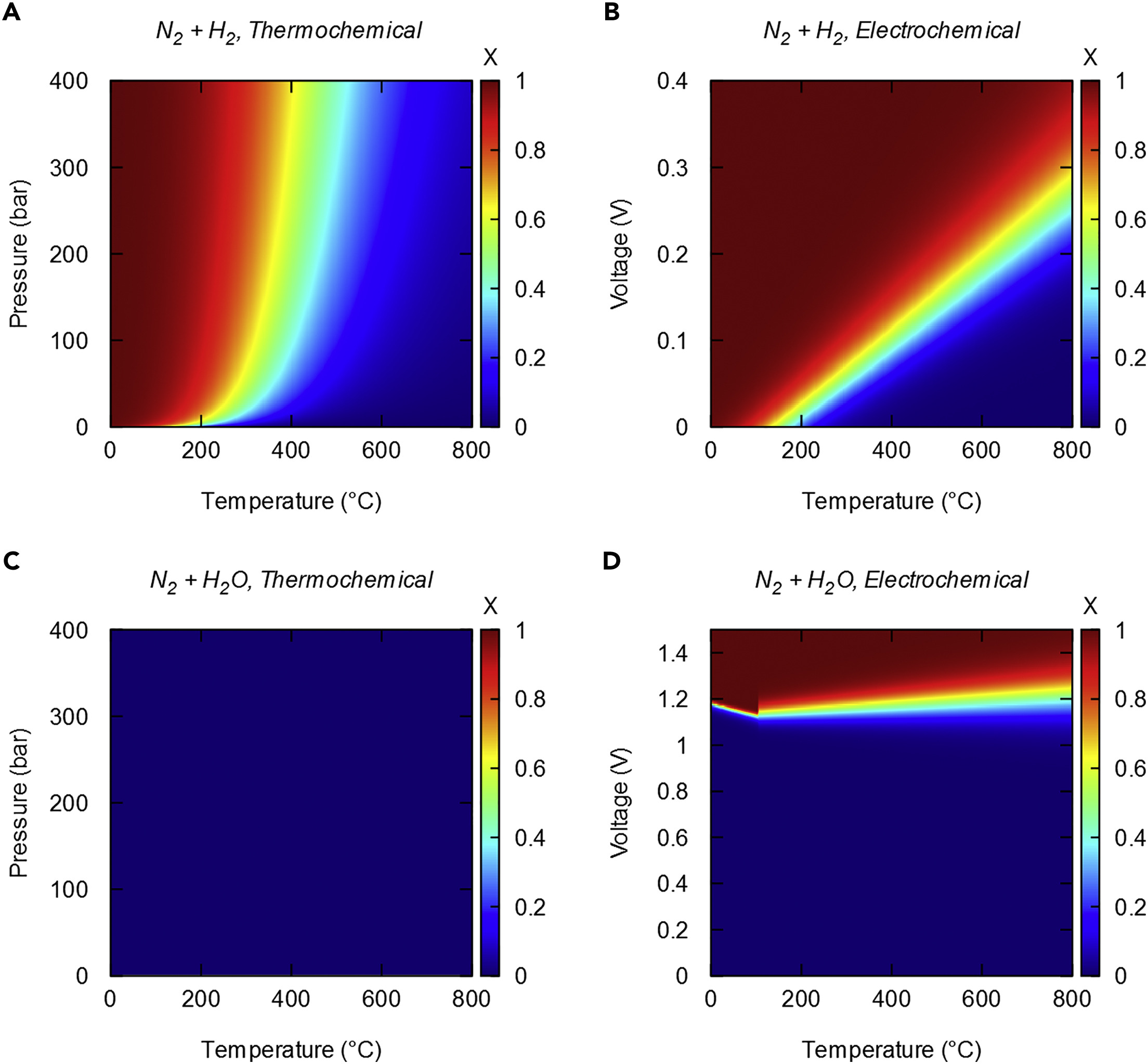
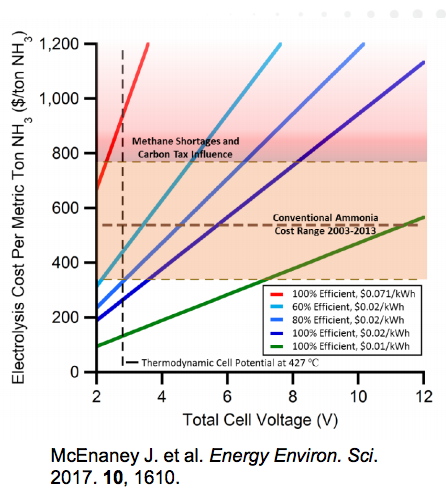
Ammonia Energy Coming on Like Gangbusters in Australia
NH3FA.Oz, the Australian chapter of the NH3 Fuel Association, held a meeting on August 30 in approximate observance of its one-year anniversary. John Mott, one of the founders of NH3FA.Oz and a member of the NH3 Fuel Association’s Advisory Board, reported that more than two dozen stakeholders from academia, industry, and the public sector participated. The meeting came on the heels of the rapid-fire release of three significant reports, and preceded by a week the announcement of an important set of research grants. The meeting, the reports, and the announcement all made clear that ammonia is fast becoming a fixture in Australian energy policy.