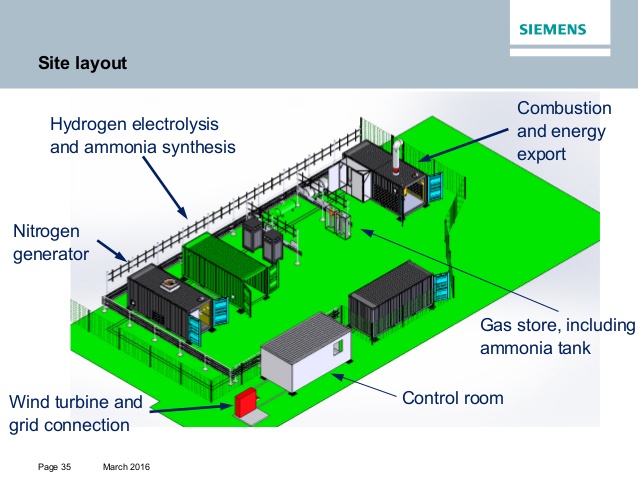
US DOE: The REFUEL Project
In April 2016, the United States Department of Energy (DOE) released a Funding Opportunity Announcement (FOA) for its Renewable Energy to Fuels through Utilization of Energy-dense Liquids (REFUEL) program. The focus of the program is carbon-neutral liquid fuels (CNLFs). In the DOE’s formulation, CNLFs are to be produced “from air and water using electrical or thermal energy from renewable sources.”