U.S. EPA’s Toxicological Review of Ammonia
By Stephen H. Crolius on January 18, 2017
On September 20 last year, the U.S. Environmental Protection Agency (EPA) announced the release of the “IRIS Toxicological Review of Ammonia – Noncancer Inhalation (Final Report)”. The “Interagency Science Discussion Draft of the Ammonia IRIS Assessment” and accompanying comments were also released. The report was the culmination of almost five years of work by the EPA and a specially convened Scientific Advisory Board. September 20 also happened to be the day of the Storage and Safety Session at the 2016 NH3 Fuel Conference. This is a striking coincidence because safety is seen as a key barrier to the adoption of ammonia as a sustainable energy carrier, and the EPA’s report is a substantial contribution to the literature of ammonia safety.
The key passage of the report’s executive summary reads as follows:
Health effects of inhaled ammonia observed at levels exceeding naturally occurring concentrations are generally limited to the respiratory tract, the site of direct contact with ammonia. Short-term inhalation exposure to high levels of ammonia in humans can cause irritation and serious burns in the mouth, lungs, and eyes. Chronic exposure to airborne ammonia can increase the risk of respiratory irritation, cough, wheezing, tightness in the chest, and impaired lung function in humans.
To fully appreciate the implications of this passage, a brief digression into the fundamentals of acid-base chemistry is necessary. Ammonia is classified as a base. Bases cause excess generation of hydroxide ions (OH-) in aqueous solutions. They are the opposite of acids, which cause excess generation of hydrogen ions (H+). In general, OH- ions prefer to be mated with H+ ions. That is why water is one of the most ubiquitous and stable chemical compounds on our planet. However, if H+ ions are not available, OH- ions will seek other chemical partners.
The “+” in “H+” means that the atom has a net positive charge, which makes it an ion, and specifically a cation. The positive charge exists because the proton, the sole constituent of a hydrogen atom’s nucleus and the inherent possessor of a positive charge, is without its customary companion, the negatively charged electron. To put it anthropomorphically, a hydrogen atom without its electron – i.e., an H+ ion – is not a happy camper. It wants to acquire an electron and is not scrupulous in its methods for doing so. This is why acids are so famously reactive. When exposed to a compound whose attachment to its own electrons is weaker than the H+ ions’ lust for them, the H+ ions attack the compound and form chemical bonds that relieve their need for electrons. For the substance that contributed its electrons, the changes that result very often read at the macroscopic level as degradation. The mirror image of this dynamic applies with bases and their drive to bond with substances that can accept their extra OH- electron.
An understanding of this chemistry is indispensable for anyone who wants to discuss the safety profile of ammonia. The hazard that ammonia presents to human beings begins and ends with its basic nature. When ammonia encounters flesh (which by definition embodies some amount of moisture), it will react with that flesh immediately and strongly, and in a way that results in degradation.
So now we can return to the conclusion of the Integrated Risk Assessment System (IRIS) report: “Health effects of inhaled ammonia observed at levels exceeding naturally occurring concentrations are generally limited to the respiratory tract, the site of direct contact with ammonia.” The effects are limited to the respiratory tract because this is typically the wettest point of initial contact between ammonia and the human body. And because, once the vaporous ammonia has dissolved in the bodily moisture and created a basic solution, the hydroxide ions react quickly with the proximate tissues. And because once this reaction occurs, the ammonia no longer has the ability to inflict further damage. It is literally neutralized. This is important because it distinguishes ammonia from the very large number of chemical agents that act on our cells and metabolism in subtler and more nefarious ways.
The U.S Occupational Safety and Health Administration (OSHA) groups a broad set of work-place safety concerns under the heading Chemical Hazards and Toxic Substances. According to the agency, “chemical hazards and toxic substances pose a wide range of health hazards (such as irritation, sensitization, and carcinogenicity) and physical hazards (such as flammability, corrosion, and explosibility).” To this list of charges, ammonia pleads guilty to irritation and corrosion. The degradation of tissue by ammonia is manifested first as irritation and then, if it progresses far enough, as a chemical burn (corrosion of the flesh).
Ammonia is innocent of the other charges. It is not a sensitizer, which is defined by OSHA as “a chemical that causes a substantial proportion of exposed people or animals to develop an allergic reaction in normal tissue after repeated exposure to the chemical.”
The IRIS report did not consider ammonia as a possible carcinogen, presumably because, according to the U.S. Centers for Disease Control, “There is no evidence that ammonia causes cancer.” In fact, evidence that ammonia causes cancer is extremely unlikely to ever materialize because, while carcinogens insinuate themselves into the reproductive machinery of the cell, ammonia at high concentrations destroys the cell from the outside in. And at low concentrations, it is present within and outside the cell as a normal part of mammalian metabolism.
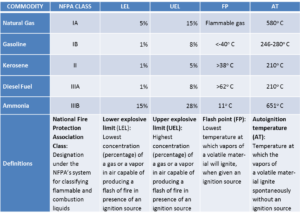
Relative to flammability and explosibility, ammonia resides in category IIIB in the categorization scheme used by the National Fire Protection Association and Federal agencies in the U.S. IIIB is the least hazardous category. Another substance found in category IIIB is vegetable oil. Natural gas, gasoline, and diesel fuel are found respectively in categories IA, IB, and IIIA.
So while it might seem extreme for the EPA to have spent multiple years studying the non-cancer effects of ammonia, and to have published its findings in a 99-page report (with accompanying volumes), the implications for ammonia as an energy carrier are clear and striking. Ammonia is hazardous. This is universally accepted. But it is hazardous in very specific ways and under very specific circumstances. The ammonia industry knows the parameters of ammonia hazard, and has been working to address them through organized, industry-wide efforts with the initiation of the American Institute of Chemical Engineers’ annual Ammonia Safety Symposia in 1956 and the subsequent chartering of national safety bodies such as the United States’ Ammonia Safety Training Institute.
The industry has shown that if a regime is in place to manage the parameters of hazard, an admirable record of safety can be achieved. As evidence, consider the safety record of the ammonia-intensive refrigeration and air-conditioning service and repair industry. OSHA’s database of Fatality and Catastrophe Investigation Summaries contains 115 “events” that have been investigated in the industry since 1985. It is a terrible litany of workers falling through skylights and being electrocuted by unsuspected live conductors. When it comes to ammonia, though, only one event is listed. Workers went into a compressor room to investigate an ammonia leak. They were not wearing adequate personal protective equipment. They suffered chemical burns. No one died.
The safety profile of ammonia specifically as an energy carrier has been studied on at least two recent occasions. In 2005, Risø National Laboratory in Denmark produced Safety Assessment of Ammonia as a Transport Fuel. In 2009, Quest Consultants produced Comparative Quantitative Risk Analysis of Motor Gasoline, LPG, and Anhydrous Ammonia as an Automotive Fuel under contract to Iowa State University.