Panel discussion on next-generation ammonia synthesis
By Kevin Rouwenhorst on December 03, 2020
Editor’s note: Kevin Rouwenhorst is a PhD researcher at the University of Twente. After his MSc thesis on power-to-ammonia-to-power, he started his PhD research on plasma-catalytic nitrogen fixation in 2018. He has published various articles, among which is a recent review on plasma-catalytic ammonia synthesis.
This year’s Ammonia Energy Conference included a panel discussion on next-generation ammonia synthesis, moderated by Sarb Giddey (CSIRO, Australia), and featuring panelists Doug MacFarlane (Monash University, Australia), Karthish Manthiram (MIT, United States), and Michael Stoukides (Aristotle University of Thessaloniki, Greece).
The panel discussed the direct fixation of nitrogen in the form of ammonia from water and air in a single electrochemical device, which is considered the “holy grail” of ammonia synthesis. Due to growing awareness of ammonia’s potential role in decarbonizing society, this topic has recently received intense attention, with hundreds of research reports published in recent years. During the panel, the participants gave their perspectives on the state of the art, and the obstacles and opportunities for progress.
The promise of electrochemical ammonia synthesis
In case of electrochemical ammonia synthesis, ammonia is formed by applying a potential over an electrochemical cell with a catalyst. This technology has the potential to lower the energy consumption of ammonia production. In case of the electrolysis-based Haber-Bosch process, water is split to hydrogen and oxygen in an electrolyzer, and subsequently the hydrogen is combined with nitrogen to form ammonia. During the process to form ammonia from hydrogen and nitrogen, heat is released. Therefore, the theoretical minimum energy consumption for an electrolysis-based Haber-Bosch process is 21.3 MJ per kg ammonia formed. On the other hand, in case of electrochemical ammonia synthesis, the proposed reaction pathway is to form ammonia directly from water and air. Therefore, the theoretical minimum energy consumption for the electrochemical ammonia synthesis process is 18.6 MJ per kg ammonia formed. Furthermore, using a single electrochemical device may result in a lower capital investment as compared to the conventional technology.
The difficulty of electrocatalytic ammonia synthesis
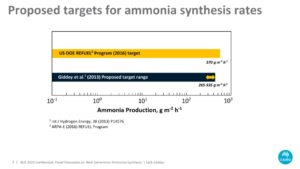
Sarb Giddey, provided an estimate of the reaction rates and energy efficiencies required for commercialization of electrochemical ammonia production technologies: reaction rates of at least 265-535 g ammonia m-2 h-1 at >50% Faradaic efficiency.
Current reported synthesis rates for direct electrocatalytic ammonia synthesis are far below this target, at least two orders of magnitude lower at Faradaic efficiencies up to 20%. Thus, the direct formation of ammonia from air and water in an electrochemical device remains an unsolved scientific challenge.

On top of this, seemingly promising results that have been reported over the past decades are increasingly being challenged by more rigorous studies that identify and eliminate false positives, including recent work by the group of Doug MacFarlane. Due to the low electrochemical ammonia synthesis rates and the low ammonia concentrations reported, background contamination typically results in false positives. Materials used in laboratories, such as gloves and catalyst materials, may contain ammonia. Furthermore, tap water typically contains various contaminations, including ammonium ions and other nitrogen oxides. Furthermore, nitrogen oxides may react to form ammonia, leading to potentially misleading results. Therefore, highly purified water without nitrogen contaminants is required, as well as isotope labelling studies and dedicated analysis techniques.
It is not surprising that electrochemical ammonia synthesis under ambient conditions is not trivial. As Michael Stoukides remarked: we spent about a decade to go from 500-550°C to 350-400°C. Only recently, novel ammonia synthesis catalysts have emerged, which form ammonia under milder conditions of 250-350°C. Stoukides also positively remarked that the large number of excellent researchers could lead to a breakthrough in research.
Other strategies to form ammonia
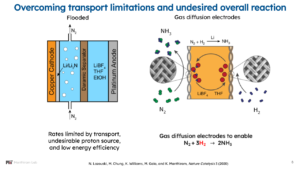
Thus, the direct electrocatalytic ammonia synthesis indeed remains difficult to achieve. However, scientists have been innovative in circumventing difficulties in electrochemical ammonia synthesis. One way to overcome this difficulty is a chemical looping approach, for example via a lithium-lithium nitride system. In the group of Karthish Manthiram at MIT, research on lithium-lithium nitride systems is also conducted with innovative anode and cathode design.
A benefit of such lithium-mediated systems is the higher reported ammonia synthesis rates of about 10 mA cm-2, as compared to rates below 0.1 mA cm-2 typically reported for the direct electrocatalytic ammonia synthesis. However, energy efficiencies reported for lithium-mediated systems are currently as low as 2%. Thus, plenty of progress remains for electrochemical ammonia synthesis, awaiting the genius of many chemists.
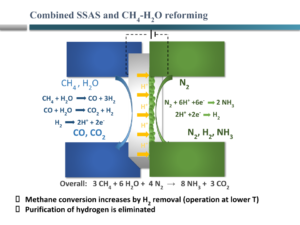
A recent approach of the group of Michael Stoukides, one of the pioneers in electrochemical ammonia synthesis, is to combine various steps in conventional ammonia synthesis in a single device. Recently, his group reported on an electrochemical Haber-Bosch system that combined all steps, from methane input to ammonia synthesis, in a single electrochemical device using high temperature solid oxides. However, ammonia synthesis rates using this technology also have ammonia synthesis rates as low as 1.2 g ammonia m-2 h-1 at 5.5% Faradaic efficiency (well below the Giddey benchmark of 265-535 g ammonia m-2 h-1 at >50% Faradaic efficiency).
All in all, electrochemical ammonia synthesis remains a matter of research, and near-term commercialization seems highly unlikely. However, many researchers remain dedicated, and all the panelists agreed that there is potential for substantial progress in the coming decades, and an important long-term role for electrochemical synthesis in the roadmap to an ammonia economy.
Full copies of these conference presentations will be available as PDFs to download from this website in the coming days. You can view the archive video of this panel discussion and all the other sessions, as by registering for the Ammonia Energy Conference 2020.