Technology status: alkaline electrolysis for renewable ammonia production
By Kevin Rouwenhorst on August 02, 2023
Renewable ammonia has been produced via alkaline electrolyzers for over a century. With the emergence of low cost solar PV and wind power, increased capacities of electrolyzers will be required. Other electrolyzers technologies are scaling up capacity, such as proton exchange membrane (PEM) electrolyzers and solid oxide electrolyzers, while anion exchange membrane (AEM) technology is now emerging. However, alkaline electrolyzers are still expected to play a significant role for renewable ammonia production.
Historical developments: electrocatalysts & stack design
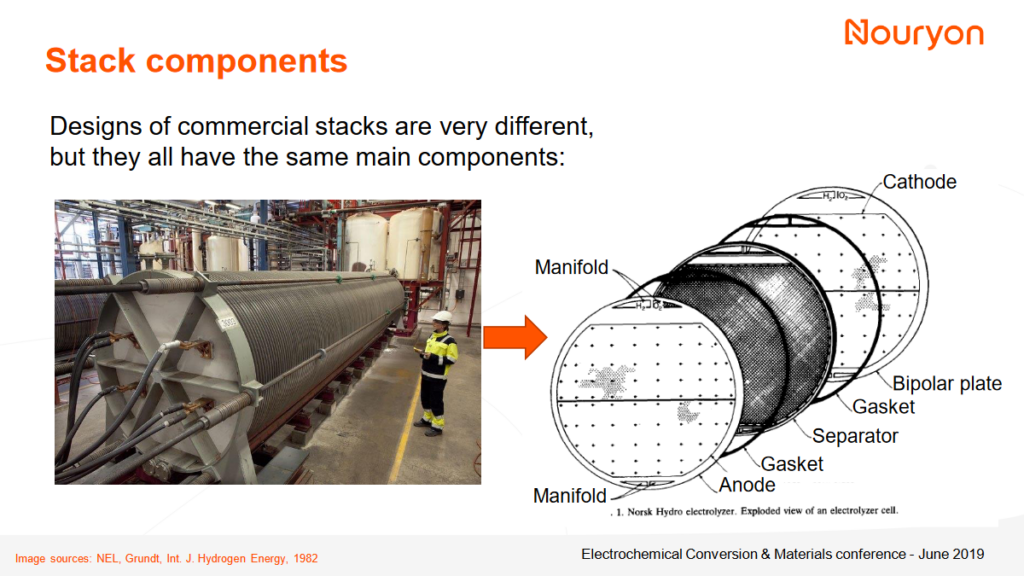
An alkaline electrolyzer operates with two metallic electrodes (cathode and anode) immersed in an alkaline aqueous electrolyte (typically 20-40 wt. % KOH in water). A diaphragm separates the electrodes, such that hydroxide ions (OH–) can be transported from the cathode to the anode. Overall, 2 hydrogen (H2) molecules and 1 oxygen (O2) molecule are produced from 2 water (H2O) molecules in the cell.
In practice, the power requirement for the electrolyzer is higher than the theoretical power requirement to split water. This is due to “imperfect” electrocatalysts, which then lose further efficiency when heated, as well as resistance in the electrolyte solution & the diaphragm.
Throughout the decades, various improvements have been implemented. For example, the Asbestos diaphragm (2-4 mm thick) was replaced by a thinner polysulfone-ZrO2 diaphragm (Zirfon®, 0.5 mm), thereby reducing efficiency losses due to the resistance of the diaphragm by nearly an order of magnitude.
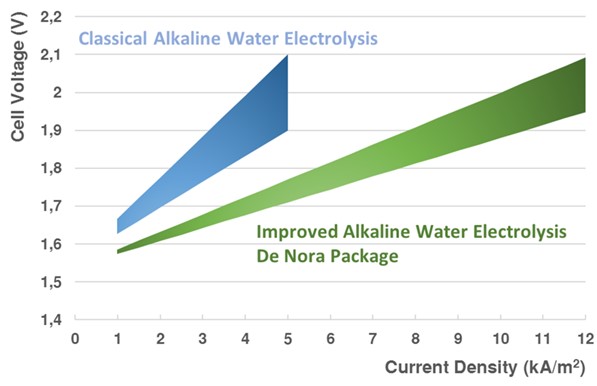
Another development is the design of more efficient electrocatalysts. An example is the development of better hydrogen evolution catalysts (cathode). For example, Japanese electrolyzer manufacturer Asahi Kasei developed new hydrogen evolution catalysts. Rather than using metallic Nickel as electrocatalyst, the cathode is replaced with Ruthenium supported on a ceria and a nickel substrate. Similarly, Italian electrode manufacturer DeNora has developed noble metal-based electrodes, which allow electrolyzer manufacturers ThyssenKrupp Nucera and McPhy to operate their electrolyzer stacks at double the current density (6-9 kA/m2) compared to other manufacturers at the same energy efficiency for hydrogen production (2-4 kA/m2). This implies less stacks are required for the same hydrogen output, which requires a higher investment per stack due to noble metal loading.
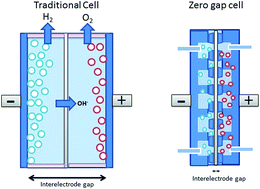
Lastly, the stack design has been successfully optimized over time. Historically, the electrodes were immersed in the electrolyte solution, and were not in direct contact with the diaphragm. Therefore, transport of hydroxide ions through the electrolyte caused significant resistances and thus heat losses. Upon innovating the cell design with the cathode and anode in contact with the diaphragm in a ‘zero gap cell’, these transport resistances are lower, resulting in a better cell efficiency.
The effects of pressure on flexibility
Typical commercial alkaline electrolyzer stacks operate at a power requirement of 3.9-4.5 kWh/Nm3-H2, with some systems operating at atmospheric pressure and other systems operating at pressures up to 30 bar. For reference, the current cost of NEL alkaline electrolyzer stacks is about 225 €/kW (for a 200 MW purchase order). Current stacks typically have a size of 1-5 MW, and further scale-up to 20 MW is expected to minimize manufacturing costs.
For ammonia production, pressurized alkaline electrolyzers operating at 10-30 bar do not require a compression step before combining their product hydrogen with nitrogen from an air separation unit. Compared to atmospheric alkaline electrolyzers, this could save 0.1-0.2 kWh/Nm3-H2 for compression. On the other hand, increased operating pressures does result in higher crossover of hydrogen from the cathode side to the anode side, which can become especially problematic at low load operation (causes an explosive gaseous mixture to aggregate). Thus, pressurized alkaline electrolyzers can only operate down to 40% of nominal load. Atmospheric pressure electrolyzers can operate down to 15% of nominal load. It should be noted that operating compressors with variable load is not trivial.
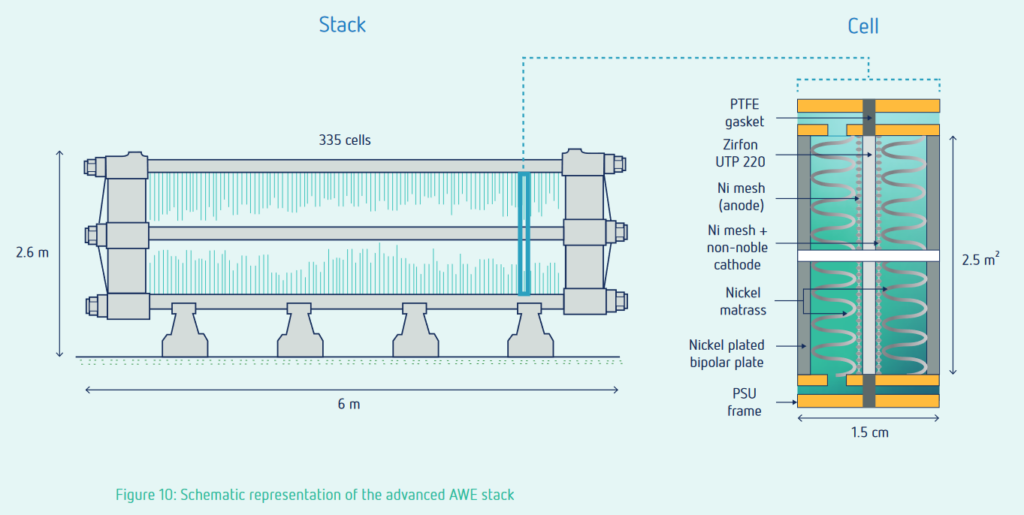
Advances in alkaline electrolyzers may allow for more flexible operation. According to De Groot and co-workers, the compromise between pressure and minimum load can be found at about 8 bar, resulting in 10% minimum load for an advanced alkaline electrolyzer. The minimum load of electrolyzers is especially relevant for off-grid renewable ammonia hubs, such as AMUN in Mauritania.