Technology status: PEM electrolysis for renewable ammonia production
By Kevin Rouwenhorst on August 01, 2023
Even though alkaline electrolysis remains the dominant technology for renewable ammonia production, PEM electrolysis offers substantial benefits like flexible operation and more compact stacks. By the end of 2023, 36% of installed electrolysis capacity dedicated to ammonia production will be based on PEM technology.
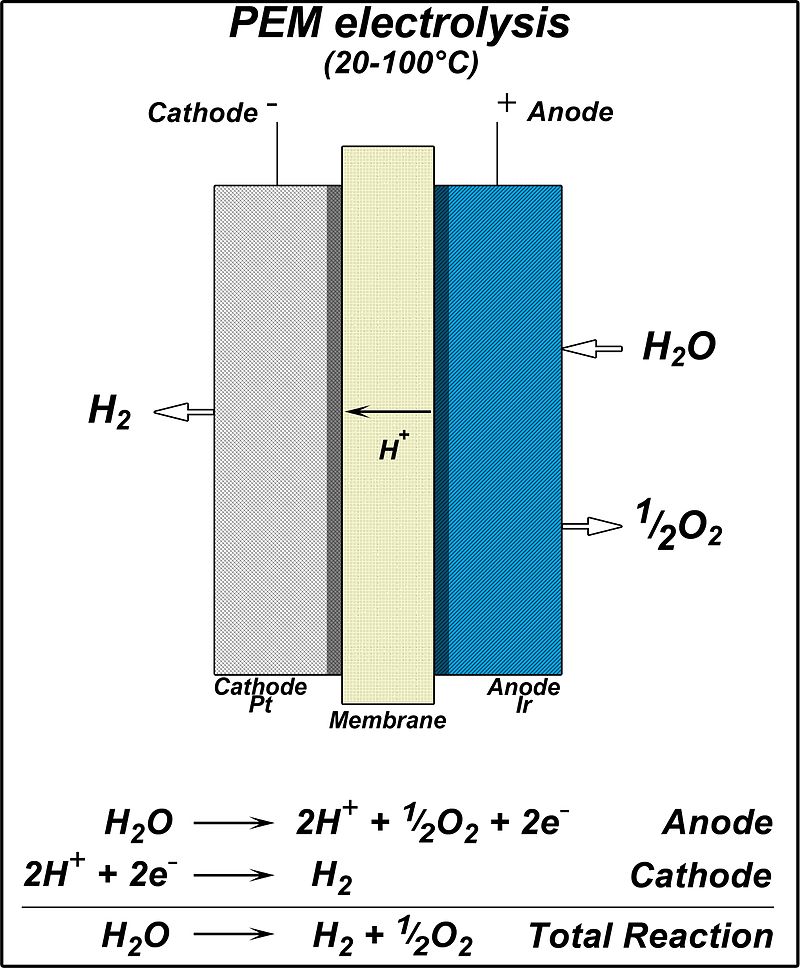
Within a PEM electrolyzer, oxygen (O2) is produced from water (H2O) at the anode side via an Iridium oxide (or Ruthenium oxide) catalyst, producing protons (H+) and electrons (e–) in the process. The protons pass through the proton exchange membrane (PEM, Nafion™), and hydrogen (H2) is produced on the cathode side via a Platinum (or Platinum-Palladium alloy) catalyst.
Recent PEM developments have centered around two issues: i) the relative scarcity of Iridium, which can be a limiting factor for scaling up PEM electrolyzer manufacturing, and ii) the membrane thickness, which limits the efficiency of the PEM electrolyzer.
Iridium loading as determining factor for scale-up
Iridium is one of the scarcest metals, and is a byproduct of platinum mining. For every tonne of platinum obtained from ores, about 39 kilograms of iridium is produced. Iridium is used in LED technologies as well as electrochemical processes (such as PEM electrolysis). In 2021, the total global iridium production was just 7.9 tonnes, of which 89% was produced in South Africa. The remaining production is in Zimbabwe and Russia.
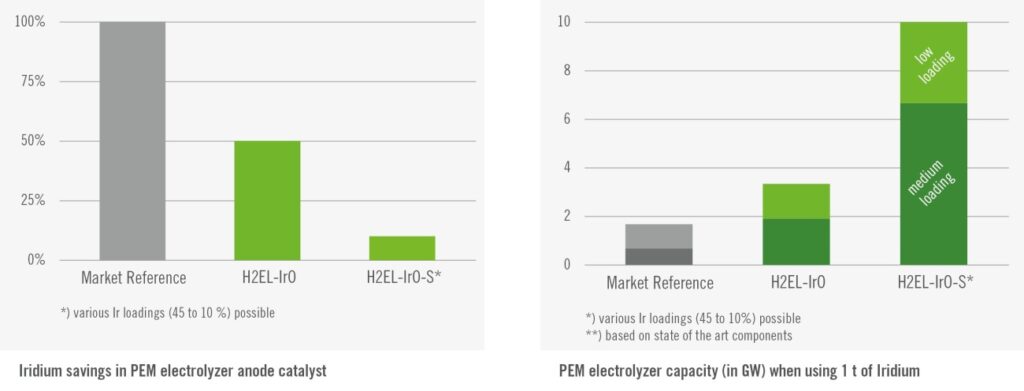
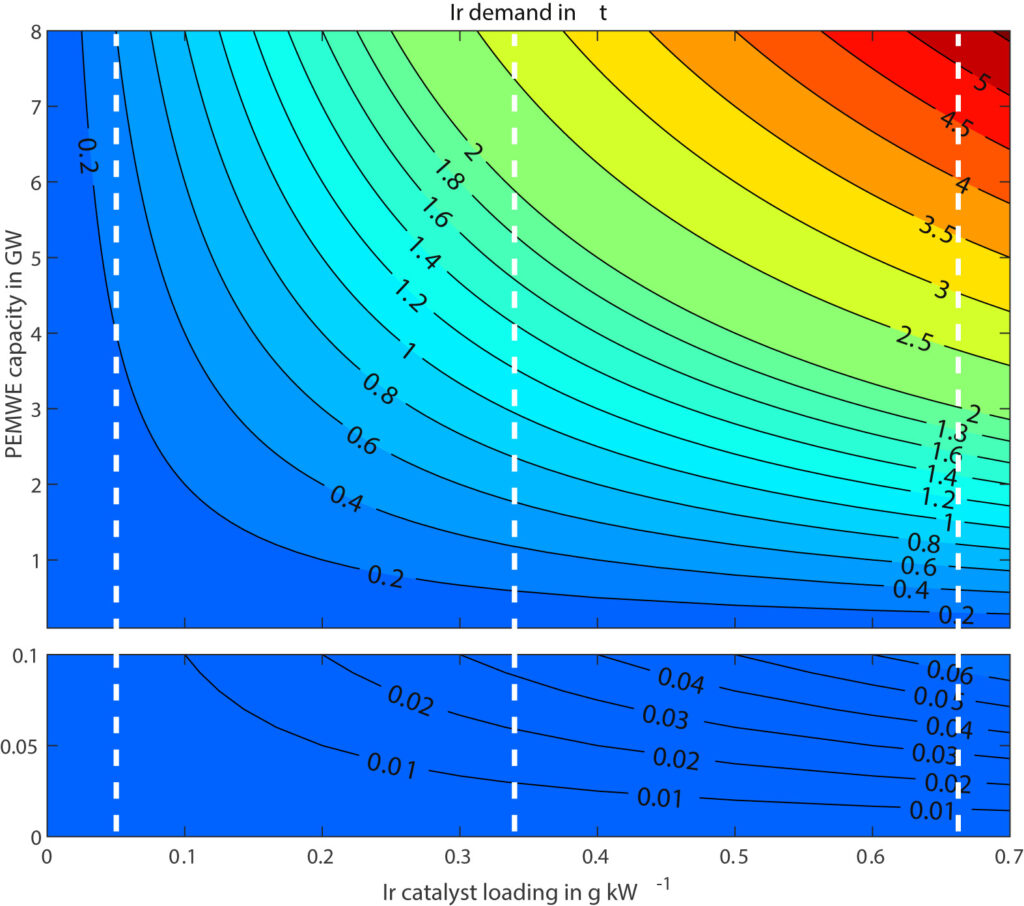
The global manufacturing capacity for PEM electrolysis is 1.55 GW per annum, according to the IEA. Minke and co-workers estimated that about 0.67 gram Iridium is currently required per kW PEM electrolyzer. If all manufacturing capacity for PEM electrolysis is utilized, this would translate to an Iridium requirement of 1.0 tonne, or 13% of global Iridium production capacity. Expansion of PEM electrolysis manufacturing capacity is only possible if Iridium loading is reduced, without compromising the catalyst activity. In the longer term, recycling of Iridium from end-of-life PEM electrolysis stacks is required to minimize additional Iridium requirements.
Precious metal company Heraeus has already commercialized Iridium catalysts (H2EL-IrO) with a reduced load down to 0.30 gram Iridium per kW. This would reduce the Iridium requirement to 0.5 tonnes or 6% of global Iridium mining capacity. For reference, Hereaus estimates that about 1-2 gram Iridium was required per kW PEM electrolyzer before 2020.
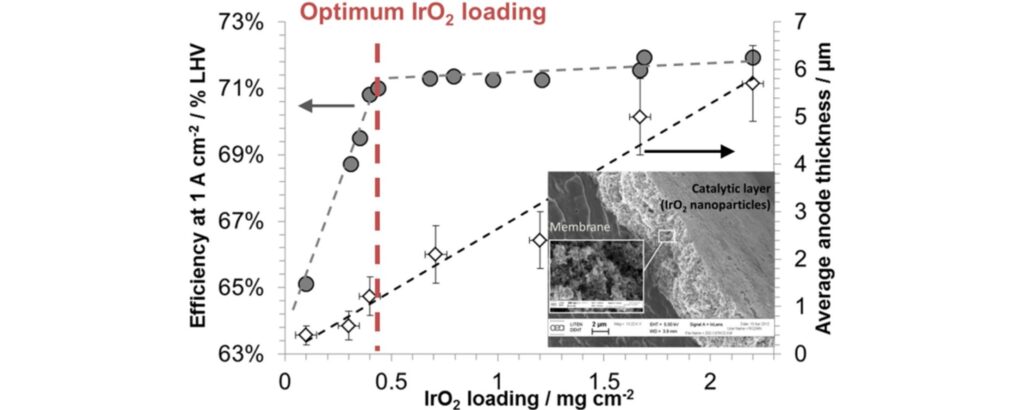
Further reduction in Iridium loading is actively studied in Universities and research institutes. Rozain and co-workers found that the performance for PEM electrolysis was not compromised down to 0.13 gram Iridium per kW. Möckl and co-workers have tested Iridium catalysts with loadings as low as 0.1 gram iridium per kW in long duration tests. Dutch research institute TNO claims a 200 times reduction of Iridium loading, while retaining a third of the performance compared to current PEM electrolyzers. Shi and co-workers recently demonstrated Ruthenium oxide as an alternative for Iridium.
Los Angeles-based PEM electrolysis start-up H2U Technologies has demonstrated a completely Iridium free electrode for its PEM electrolyzer, based on technology developed at the California Institute of Technology.
Hystar: reducing the membrane thickness
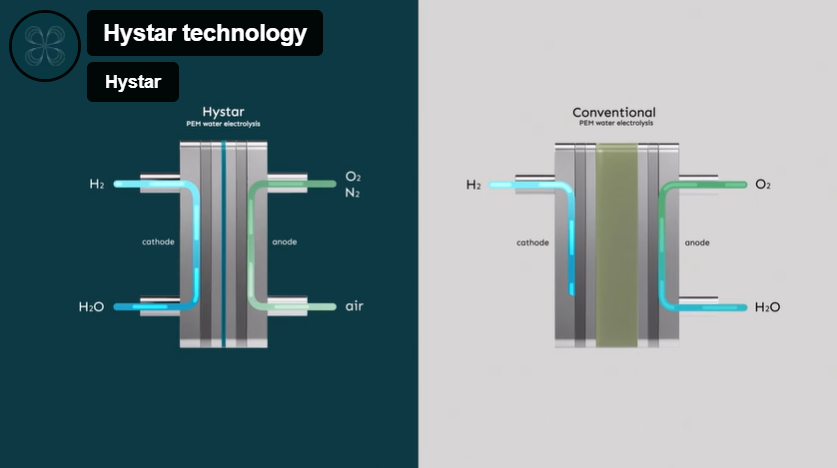
HyStar is a Norwegian start-up and spinoff from SINTEF, which has developed a novel PEM electrolysis cell configuration. The performance of conventional PEM electrolysis is limited due to the thickness of the proton exchange membrane (about 175 μm), causing resistance to proton flow and therefore heat losses. However, this thickness is required to prevent H2 crossover from the cathode to the anode side, which could result in an explosive mixture for thinner membranes.
Hystar reduces this risk by introducing humidified air on the anode side, instead of water. This results in strongly diluted oxygen in a nitrogen atmosphere, reducing the risk of an explosive mixture. Therefore, Hystar can utilize a thinner membrane (20 μm). This allows the electrolyzer to operate at 2.5 times the current density compared to a conventional PEM electrolyzer.