The Ammonia Academic Wrap: “seamless” cracking, improving Haber Bosch, a novel green power-to-ammonia-to-power solution and a review into the use of ammonia as a fuel
By Julian Atchison on May 13, 2021
Welcome to the Ammonia Academic Wrap: a summary of all the latest papers, developments and emerging trends in the world of ammonia energy R&D.
“Seamless” ammonia cracking tech from Northwestern

In their recent Joule paper, researchers at Northwestern University (with assistance from SAFCell) demonstrated the production of high purity hydrogen from ammonia at intermediate temperatures (250°C), using a solid, acid-based electrochemical cell. Sossina Haile sees cracking breakthroughs like these as key to enabling the widespread use of hydrogen as transportation fuel by allowing for the safe, energy-efficient cracking of ammonia onsite at hydrogen refueling stations.
New electrolysis catalyst
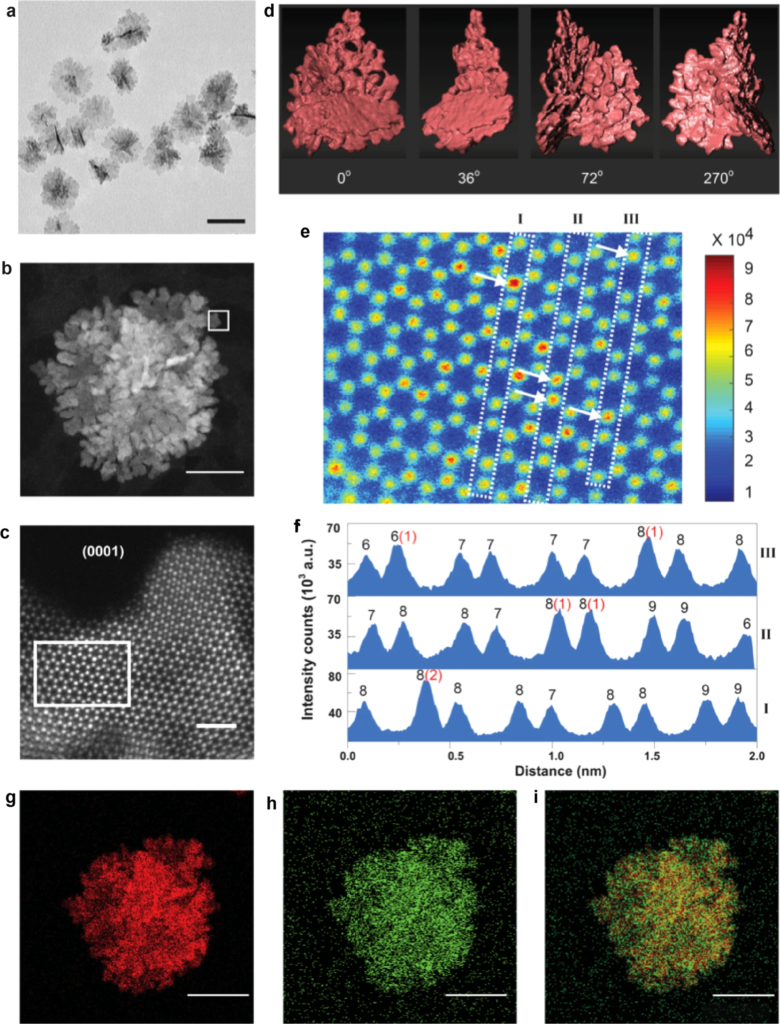
A research team at Kyoto University have demonstrated a promising new Ru-Ir catalyst for hydrogen production in a recent Nature Communications paper. Compared to traditional Ir-oxide catalysts, the new alloy can produce 30 times the amount of hydrogen in a given time period, and be used for 10 times longer than current, off-the-shelf options. The new alloy is also 94% Ru by content, giving it a distinct cost and ease-of-supply advantage over Ir and Pt based catalysts currently favored. Given the cost of water electrolysis units is still a major obstacle for their widespread deployment, reducing catalyst manufacturing costs to a fraction of their current levels would be a major boon for the industry.
Successful integration of ammonia synthesis and separation for improved efficiency
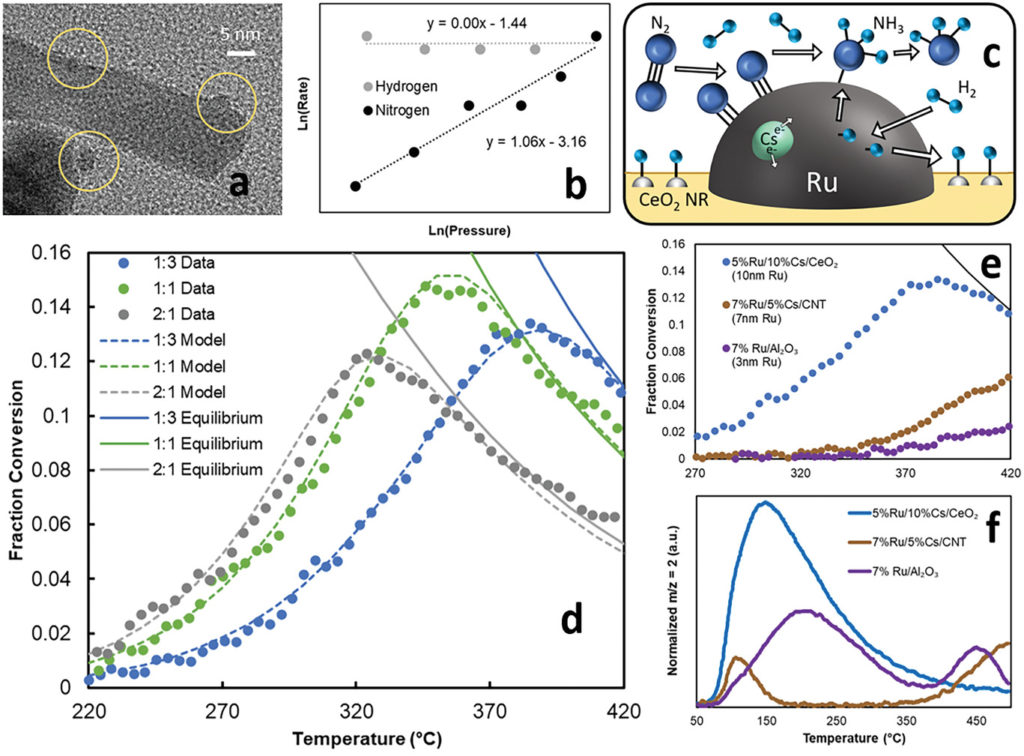
At Cambridge, researchers have combined Ru-based catalysts with a nano-scale support structure (CeO2 and Cs) to facilitate ammonia synthesis at lower temperatures and pressures than traditional Haber Bosch processes, according to their new Advanced Energy Materials paper. The two support structure materials work in tandem to remove hydrogen and ammonia inhibition effects at the catalytic surface, enabling the system to reach equilibrium quickly, and at <300°C and 20 bar (versus >400°C and 100 bar for traditional HB). When integrated with a novel adsorbent (MnCl2 supported by silica gel), overall hydrogen conversion is maximised at >90%.
More research needed into transition metal catalysts for HB
A review of current trends in HB improvements by Jimmy Faria at the University of Twente reaches a similar conclusion to the Cambridge study. But, while the Current Opinion in Green and Sustainable Chemistry paper agrees with the use of novel metal halide adsorbents like MnCl2 and the use of catalyst support structures to remove hydrogen and ammonia inhibition, the continued, widespread use of expensive, rare transition metals like Ru is not recommended.
Rather, a switch to cheap, widely available transition metal catalysts is needed, and this requires further research to ensure that a new generation of catalysts can be developed without “jeopardising” the catalytic activity and stability associated with rare transition catalysts. Further development of the catalytic support structures to maximise surface area and heat transfer is also recommended by Faria. But the efforts will be well worth it: these improvements “could potentially reduce operational and capital expenditures of the process even at small scales“, unlocking whole new possibilities for sustainable HB ammonia synthesis in a wide range of situations.
A novel, green power-to-ammonia to power system
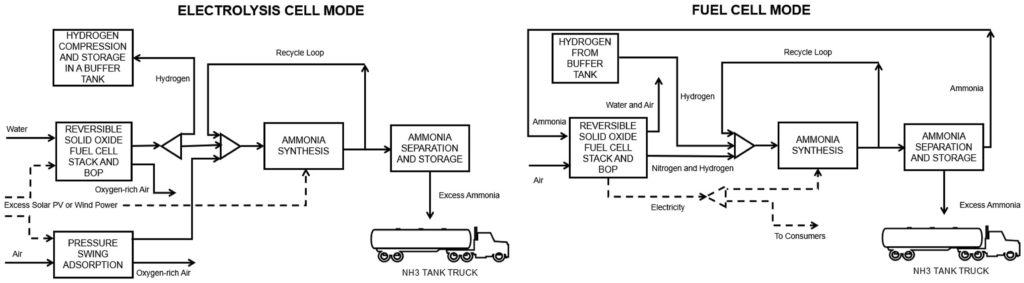
Researchers at Cranfield University (UK) have simulated a fully integrated, reversible solid oxide fuel cell (RSOFC)/Haber Bosch system that can be quickly swung in either of two directions: Fuel Cell mode or Electrolysis Cell mode. Assuming solar PV or wind energy from an adjacent source is readily available for hydrogen production, the system can achieve electricity consumption rates of 6.41 kWh/kg produced NH3 in FC mode and 8.21 kWh/kg produced NH3 in EC mode, at ammonia production efficiencies of 79%–87% — a higher overall efficiency than current large-scale ammonia synthesis setups. According to the authors of the International Journal of Hydrogen Energy paper, the system also has the distinct advantage that there is no “down time”: ammonia is always produced for market with the option to swing the system into power generation mode and provide support services to the grid or local electricity users. The system is also a highly efficient user of intermittent renewable energy resources.
Review on ammonia as a potential fuel
A review led by regular Ammonia Energy contributor Agustin Valera-Medina (Cardiff University) has identified key obstacles that need to be overcome for the widespread adoption of ammonia as a fuel, as well as promising opportunities. The Energy Fuels paper concludes that:
- in the synthesis space, “process intensification” and the use of solid oxide electrolysis could achieve further energy efficiency and consumption savings.
- ammonia engines are fast approaching commercialisation. Although more research into the combustion of ammonia and ammonia mixes is required, these problems will likely be overcome within the decade.
- legislative changes allowing the use of ammonia as fuel represent a major challenge. Currently, no jurisdiction has a legal approach that addresses both environmental and healthy & safety concerns.
- the full electrification of Haber Bosch plants using only renewable energy will be a huge economic milestone.
Sign up for weekly AEA updates
Make sure you’re signed up for AEA email updates, including our weekly newsletter featuring wrap articles just like this one.