Progress for Low-Temperature Direct Ammonia Fuel Cells
By Stephen H. Crolius on December 14, 2017
Speaking at the NH3 Energy+ Topical Conference last month, University of Delaware Adjunct Professor Shimshon Gottesfeld reported on progress made by the university’s direct ammonia fuel cell (DAFC) project. Evidently, the UDel team is now a big step closer to its goal of establishing the DAFC as a viable automotive power plant.
The project is being funded by the US Department of Energy’s Advanced Research Projects Agency – Energy (ARPA-E). Work on the three-year effort started during the summer. The extended team also includes representatives from Brookhaven National Laboratory, Rensselaer Polytechnic Institute, and the advanced materials start-up Xergy Inc.
The abstract for Gottesfeld’s presentation starts with the core argument for ammonia as a transportation fuel:
A detailed techno-economic evaluation of carbon free and carbon neutral fuels, made in the preparatory phase of this Project, revealed that the combined cost of fuel storage and fuel transport is the lowest for liquid ammonia. This is a direct result of the high energy density and the liquid form of the fuel under conditions very close to ambient.
Gottesfeld et al, Direct Ammonia Fuel Cell Utilizing an OH- Ion Conducting Membrane Electrolyte, November 2017
The next step in the intellectual journey, according to the abstract, was the decision to use polymer electrolyte membrane (PEM) chemistry. In contrast to the hydrogen-powered version of PEM fuel cells which incorporates proton exchange membranes (which also carry the acronym PEM), the ammonia-powered PEM fuel cell involves the systematic flow of hydroxide (OH-) anions. The decision to go with the polymer electrolyte membrane category was based on “the inherent advantages of this type of low temperature fuel cell in powering passenger vehicles which typically require a number of stop-restart cycles per day.” In making the decision, the UDel team believed that their expertise in electrocatalysis could overcome the key challenge of the “low rate of anodic oxidation of ammonia, reported to date for DAFCs operating at low cell temperatures.”
Gottesfeld enumerated the program’s four technical objectives in his talk:
- Peak power density of at least 50% of a direct hydrogen fuel cell
- A chemical-to-mechanical-energy conversion efficiency in excess of 40% when the fuel cell is operating at 25% of peak power
- Maximum start-up time of several minutes
- Zero tail-pipe emissions
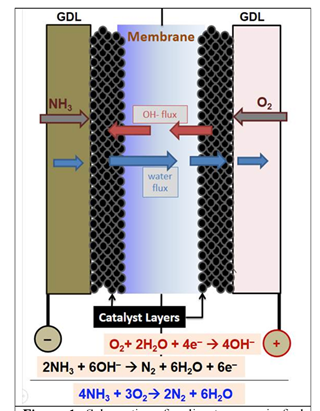
Inherent in this formulation is the key challenge addressed by the team at the project outset: the need to find a sweet spot on the fuel cell’s operating temperature gradient. Low temperatures dampen the rate of catalytic generation of hydroxide (OH-) ions from water and decomposition of ammonia into nitrogen and hydrogen. High temperatures promote degradation of the electrolyte membrane. The team initially chose 100 °C as the likeliest compromise between these two considerations. The make-or-break focus of their work will be finding a catalyst that can produce sufficient chemical reactivity and a membrane that will prove sufficiently durable at that temperature.
This search represents the context for Gottesfeld’s good news. The team has developed and integrated a set of components that in “early tests” have made positive strides. The Delaware researchers developed a bimetallic catalyst for the fuel cell’s NH3-decomposing anode and a silver catalyst for the hydroxide-producing cathode. Team members RPI and Xergy provided two types of OH- ion conducting membranes. The integrated assembly “resulted in DAFC power levels around 90 °C that were more than an order of magnitude higher than reported to date.” The team’s abstract also credited “the quality of the membrane / electrode assemblies … optimized gas flow rates and inlet RH [relative humidity] levels.”
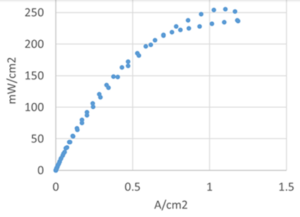
Gottesfeld said that the targeted DAFC specifications can likely be attained with an increase in operating temperature to approximately 120 °C. In his summary he confirmed that the work “has yielded promising cell performance, supporting the prospects of this fuel cell technology to become a viable power source for electric vehicles.”
A review of the recent technical literature suggests that the UDel team is alone or nearly so in its efforts to develop a low-temperature DAFC. If the team is successful, it will provide a third option for the use of ammonia in a fueling system for fuel cell vehicles (FCVs).
The first involves cracking ammonia into its atomic constituents at a hydrogen fueling station, with subsequent compression and dispensing of pure hydrogen to FCVs.
The second uses a solid oxide fuel cell (SOFC) as the on-board power plant. The Eguchi Laboratory at Kyoto University is working on a direct ammonia version of the SOFC. (Refer to the most recent Ammonia Energy post on this topic, or read Eguchi’s presentation at the NH3 Energy+ Topical Conference.) Toyota is among the companies collaborating in this effort.
It is worth noting that Nissan announced in June 2016 the development of a prototype SOFC vehicle, dubbed the e-Bio Fuel-Cell. In the months since that announcement, the company has been conducting road tests in Brazil. It is operating the vehicles on ethanol and drawing a favorable contrast between that fuel’s well-developed fueling infrastructure (at least, in Brazil) and hydrogen’s still-to-be-developed infrastructure. The British company Ceres Power is supplying the e-Bio Fuel-Cell’s power plant. According to a 2016 Fleet News article, “Ceres Power’s SteelCell [SOFC] technology . . . is ‘fuel agnostic,’ meaning vehicles with a SteelCell stack could generate electricity using petrol, diesel, natural gas or bioethanol as fuel.” In a conversation earlier this year, a high-ranking Ceres official told a representative of the NH3 Fuel Association that it would be “entirely possible” to operate the SteelCell on ammonia.
The prospects for ammonia as a transportation fuel grow ever-more compelling.