H2@Scale in California: A Role for Ammonia?
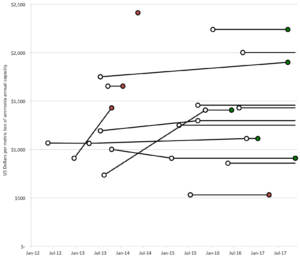
The U.S. Department of Energy H2@Scale program’s November 2017 workshop in California included mention of ammonia as a constituent of a future hydrogen economy. It also highlighted the relevance ammonia energy could have in California. California stands out globally as a large economy that is strongly committed to development of a hydrogen economy. The state’s strategy for hydrogen-powered transportation involves reducing the production cost of renewable hydrogen and the capital and operating costs of hydrogen fueling stations. It does not explicitly address the cost of intermediate hydrogen logistics. The question of cost is of utmost importance because California has so far put $120 million of public funds into hydrogen fueling stations and intends to invest an additional $20 million per year through 2022. The state’s aspiration is to move to a point where hydrogen that is used as a motor fuel is free of public subsidy. So it clearly behooves the state to investigate how ammonia could be used as a cost-reducing energy carrier. Toyota is active in California’s hydrogen movement and has announced plans to build a renewable hydrogen plant that will use cow manure as a feedstock. A project with a different conception, one that draws upon the solar and wind resources of the Mojave Desert to produce renewable hydrogen and logistically advantaged ammonia, would align better with the state’s sustainability objectives.