Round-trip Efficiency of Ammonia as a Renewable Energy Transportation Media
By Trevor Brown on October 20, 2017
A new study has made a major addition to the available literature on the economic benefits of ammonia energy. This latest study, published by researchers from CSIRO in Australia, provides the data needed to define the round-trip efficiency of using ammonia as a sustainable fuel and hydrogen carrier.
Ammonia as a Renewable Energy Transportation Media
The team at CSIRO, the Commonwealth Scientific and Industrial Research Organization, in Australia, begins its analysis of the round-trip efficiency of using ammonia as a fuel with an examination of the energy efficiency of ammonia synthesis technologies, then considers the energy cost of intermediate processes, like ammonia cracking and hydrogen storage and compression, and concludes with an assessment of fuel efficiency at the point of delivery.
At the outset, we must acknowledge the paper’s title: “Ammonia as a Renewable Energy Transportation Media.” The authors are quite clear that, relative to making ammonia, using renewable electricity directly “would clearly be far more efficient” given the distribution losses of only “less than 10%” in most electrical grids. CSIRO makes a similar case for directly charging electric vehicles because “losses during charging … are typically significantly less than 20%.”
Therefore, CSIRO provides the best argument for the use of ammonia as an energy vector:
Conversion of renewable energy to a fuel would only be considered viable if the energy is to be transported over long distances by ship, the energy needs to be stored for extended (months) periods of time or there is another engineering constraint that precludes the direct use of the generated electricity.
Giddey et al, Ammonia as a Renewable Energy Transportation Media, ACS Sustainable Chemistry & Engineering, 09/27/2017
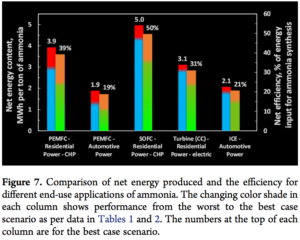
The CSIRO study specifically elucidates three end-use scenarios for ammonia fuel, quantifying the round-trip efficiency of ammonia as: (1) a high-purity hydrogen carrier for fuel cell vehicles (PEMFC), (2) a hydrogen carrier for stationary fuel cells (SOFC), and (3) a direct fuel for internal combustion engines and gas turbines.
CSIRO has published these findings as ranges, taking into account both the best-case and worst-case assumptions for the energy performance of these various technologies in various applications, including both automative and residential power (CHP and electric).
Despite the low efficiencies and different levels of maturity of the chain of technologies discussed, ammonia remains an excellent proposition for converting RE [renewable energy] to hydrogen and then to ammonia, transporting it to locations with low renewable energy intensity and converting the ammonia back to hydrogen for local consumption. The RTE [round-trip efficiency] of electrical energy storage (battery, supercapacitors) can be higher than 80%. However, the end use and generation locations have to be in close proximity.
Liquid hydrogen and methanol, despite also being alternative energy vectors, have lower RTE values [than ammonia] as estimated in previous studies. Further, the infrastructure required for liquid hydrogen transport is almost nonexistent and methanol is an emission producing fuel at the point of use; make these alternatives less attractive at this stage. Ammonia therefore provides an attractive option in terms of RTE, as well as being an emission-less energy carrier.
Giddey et al, Ammonia as a Renewable Energy Transportation Media, ACS Sustainable Chemistry & Engineering, 09/27/2017
Technical considerations
Energy content of ammonia
The CSIRO paper begins by defining ammonia as either having an energy content of 5.17 MWh per metric ton if used as a direct fuel (based on ammonia’s lower heating value, LHV), or having a hydrogen energy content of 5.91 MWh/ton if cracked back into hydrogen before use in a hydrogen fuel cell (based on hydrogen’s LHV). The increase in energy content between these two values, from ammonia’s use as a direct fuel use to its use a hydrogen carrier, would be supplied externally from the endothermic (0.75 MWh/ton ammonia) cracking reaction that separates the hydrogen (H2) from the ammonia (NH3).
Energy input required to produce ammonia
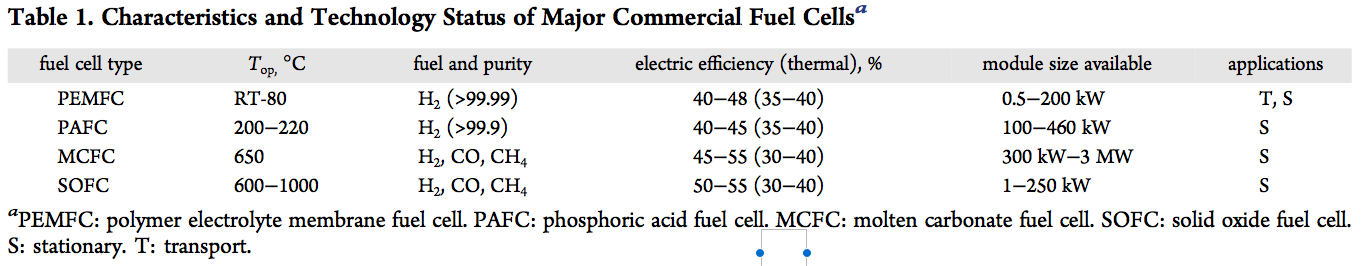
The energy input required to synthesize ammonia from fossil fuels is given as 7.8, 10.6, and 11.7 MWh/ton ammonia for feedstocks of natural gas (SMR), coal, and fuel oil respectively, or roughly 8-12 MWh/ton ammonia. These are “best practice energy consumption values … dependent on the region, plant size, and the source of hydrogen (feedstock).” The study quotes but does not incorporate in its energy assessments the associated emissions of 1.6, 3.0, and 3.8 tons CO2 per ton ammonia, respectively, for each fossil feedstock.
For renewable ammonia synthesis technologies, the CSIRO study defines an energy input range of 10-12 MWh/ton ammonia, assuming that renewable energy (solar, tidal, or wind) is used to power an electrolyzer and an ASU (air separation unit), with the resulting hydrogen and nitrogen fed into a Haber-Bosch ammonia synthesis loop.
(The authors also give us a sense of long-term technological potential. Today’s SMR-fed Haber-Bosch is more or less at its technical limit of a “best practice” 7.8 MWh/ton ammonia; CSIRO gives the “theoretical electric input required to produce hydrogen by the electrolysis of water,” as 7 MWh/ton ammonia. While we may be able to make ammonia sustainably, from renewable power, it will always be an energy-intensive process.)
With an eye on the future, the CSIRO authors also acknowledge direct electrochemical ammonia synthesis technologies, which might one day replace Haber-Bosch, but their assumptions do not rely upon the commercialization of these technologies because the energy efficiencies demonstrated thus far “need to be improved at least by 1−2 orders of magnitude to allow for commercial use.” And this team should know: the lead author, Sarb Giddey, was the influential originator of the Giddey Commercial Benchmark for electrochemical ammonia synthesis technologies.
Energy Cost of NH3 Cracking / H2 Compression & Storage
Trace amounts of ammonia in a hydrogen fuel supply would “irreversibly damage” a PEMFC. If ammonia is under consideration as a hydrogen carrier for these fuel cells, therefore, high-purity cracking technologies “require specific attention.” (This is the argument driving CSIRO’s development of its own hydrogen purification membrane.)
In this study, “the net energy required for the ammonia cracking has been calculated to be 0.28 and 0.30 MWh per ton ammonia,” for best and worst case scenarios. This is in addition to the 15% “loss of hydrogen” in cracking, equal to 1.13 MWh/ton ammonia. The ammonia cracker, therefore, leads to total losses “estimated to be 1.41 MWh per ton (equates to overall ammonia cracker efficiency 76%) for best case scenario.”
Likewise, the study considers hydrogen compression and storage technologies – after all, this RTE assessment of ammonia as a hydrogen carrier is only relevant in relation to the RTE of hydrogen itself; the study also considers methanol, as an alternative synthetic fuel. The hydrogen compression technologies considered include mechanical compression (40-50% efficient), electrochemical compression (potentially 70-80% efficient), and chemical compression using metal hydrides (less than 30% efficient, but offering the “opportunity to utilize waste heat, where available”).
For example, in the PEMFC vehicle scenario, hydrogen compression losses (including precooling) amount to “12−17% which equates to compression related losses of 0.54 – 0.67 MWh per ton ammonia.”
Theoretical results
Scenario 1: High Purity Hydrogen Production for Use in Fuel Cell Vehicles
Assuming that ammonia is produced from completely renewable power, cracked into high-purity hydrogen, and used in a PEMFC to power a vehicle, the “net efficiency for worst and best case scenario … is between 11% and 19%.”
For comparison, although the CSIRO paper does not refer to contemporary fossil-fueled cars, the gasoline or diesel vehicle equivalent of this same round-trip efficiency calculation is a carbon-fueled well-to-wheel efficiency of roughly 20%.
Scenario 2: Ammonia Utilization for Stationary Applications via Fuel Cells
For the best and worst case technologies considered for stationary power fuel cells, the “net combined heat and power (CHP) efficiency … was 25−39% … Thus, starting with one MWh renewable energy input, the net energy delivered to the grid or a distributed site for the best case scenario would be 214 kWh in the form of electricity and 179 kWh low grade heat.”
This was the highest-efficiency use-case identified in this study.
Scenario 3: Ammonia Combustion in a Turbine (Stationary) or Modified IC Engine (Transport)
The CSIRO paper provides overall efficiency data for ammonia in internal combustion engines (ICEs) (35-40%) and combined cycle gas turbines (55-60%), from which it derives best- and worst-case RTE value ranges from 15-21% in ICEs and, in turbines, “24 to 31% (electric) of the input renewable energy depending on the hydrogen production system used.”
As I have mentioned, these apparently “low” values relate to an incumbent energy system based upon a ~20% well-to-wheel gasoline-fueled internal combustion engine.
The CSIRO paper compares these ammonia data against “a number of studies investigating the round trip efficiency of methanol and liquid hydrogen pathways” for alternative fuel uses, and concludes that “neither liquid hydrogen nor methanol offer a significant efficiency gain in most cases … [for example, according to one paper] the overall efficiency was 9.3% for methanol and 8.7% for liquid hydrogen vectors.”