Catalysts
Fast-Ramping Reactor for CO2-Free NH3 Synthesis
Novel Catalysts for Ammonia Cracking and Synthesis
Delivering Clean Hydrogen Fuel from Ammonia Using Metal Membranes
Development of Materials and Systems for Ammonia-Fueled Solid Oxide Fuel Cells
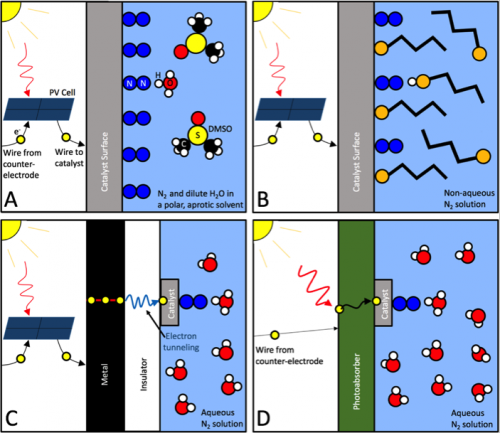
Overcoming the Selectivity Challenge in Electrochemical Ammonia Synthesis
In the last 12 months ... The research community has made great progress toward solving the "selectivity challenge" in electrochemical ammonia synthesis. Although, rather than an actual solution, mostly what we have is a range of sophisticated work-arounds that succeed in making this problem moot.
The Ammonia Economy at the ACS National Meeting
The American Chemical Society (ACS) has published the program for its 2017 National Meeting, which takes place next month in Washington DC and includes a session dedicated to the "Ammonia Economy." The first day of the week-long meeting, Sunday August 20th, will feature a full morning of technical papers from the US, UK, and Japan, covering ammonia energy topics across three general areas: producing hydrogen from ammonia, developing new catalysts for ammonia synthesis and oxidation, and storing ammonia in solid chemical form.
Progress in Ammonia Combustion Catalysts
On February 14 the Journal of Physical Chemistry published a paper entitled “Local Structures and Catalytic Ammonia Combustion Properties of Copper Oxides and Silver Supported on Aluminum Oxides.” The paper, by Satoshi Hinokuma of Kumamoto University in Kumamoto, Japan and four co-authors, reports on a catalyst system that is well adapted for use in ammonia energy applications.
International R&D on sustainable ammonia synthesis technologies
Over the last few weeks, I've written extensively about sustainable ammonia synthesis projects funded by the US Department of Energy (DOE). While these projects are important, the US has no monopoly on technology development. Indeed, given the current uncertainty regarding energy policy under the Trump administration, the US may be at risk of stepping away from its assumed role as an industry leader in this area. This article introduces seven international projects, representing research coming out of eight countries spread across four continents. These projects span the breadth of next-generation ammonia synthesis research, from nanotechnology and electrocatalysis to plasmas and ionic liquids.