TU Delft’s Battery-Electrolyzer Technology
By Stephen H. Crolius on January 13, 2017
On December 14, the journal Energy & Environmental Science published an article on a new technology, Efficient electricity storage with a battolyser, an integrated Ni–Fe battery and electrolyser. The lead author is Fokko Mulder, Professor of Materials for Energy Conversion & Storage at the Delft University of Technology (TU Delft) in the Netherlands.
The system developed by Mulder and his collaborators accepts electricity from an external source and stores it in the conventional manner of all batteries. The twist is that when the battery is fully charged, any additional incoming electricity is used to generate hydrogen and oxygen via electrolysis. The technology may prove to be a valuable element in a grid-scale ammonia-based energy system.
The battery chemistry used by Mulder and his colleagues is nickel-iron (Ni-Fe). Battery specialists with a sense of history are no doubt amused to see this chemistry appear in this 21st century context. Ni-Fe batteries had a moment of prominence in the early 1900s, having been developed and patented by Thomas Edison and proposed by him as the energy storage system for the first generation of electric vehicles.
Edison extolled the positives of Ni-Fe. In the words of Wikipedia, the chemistry produces “a very robust battery which is tolerant of abuse, (overcharge, overdischarge, and short-circuiting) and can have very long life even if so treated. It is often used in backup situations where it can be continuously charged and can last for more than 20 years.”
With all of these advantages, why didn’t Ni-Fe become one of the major battery chemistries over the last century? The answer probably relates to its less desirable properties, such as relatively low energy density, that would be problematic in automotive applications. One factor that definitely cast a shadow was the tendency of the batteries to generate hydrogen when they were being recharged. This was seen as a hazard.
The reason hydrogen is generated is because when electricity passes through the electrodes of a Ni-Fe battery, nickel oxide hydroxide (NiOOH) and reduced iron are produced. These substances serve as catalysts for the electrolysis of water.
Starting with the insight that in the context of a hydrogen energy economy, H2 generation could be a feature rather than a bug, Mulder’s group at TU Delft created a bench-scale prototype of a Ni-Fe battery from which hydrogen could be collected in an efficient and safe manner. As reported on the TU Delft Web site, success came quickly. “It worked straight away,” Mulder said. “As soon as the battery approached full charge, it started producing hydrogen.”
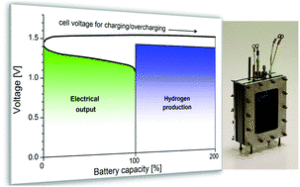
The group has since determined that the system’s performance on efficiency and durability dimensions is promising. “By combining battery technology with electrolysis, we achieve an outstanding overall efficiency of up to 90%,” Mulder said. “The battolyser has also been found to be stable, both in battery and electrolysis mode, even under long, intensive charging, discharging and hydrogen production.”
Electricity is a real-time energy commodity. Demand must be met instantaneously with supply. Supply must instantaneously meet demand. Costly resources are deployed to ensure that this balance is maintained. The challenge becomes more severe as renewable resources grow as a part of the generating mix. Now, not only is demand constantly fluctuating, supply is too. Regulators in leading jurisdictions are mandating the deployment of energy storage technologies as the proportion of renewable generation goes up.
The real-time nature of electricity also creates an opportunity in cases when electricity can be generated inexpensively but cannot be used in the moment. As described in a recent post, “Carbon Pricing and the Economics of Green Ammonia”, such electricity is said to be “curtailed, spilled, or constrained”. Examples are hydropower generators during seasons of abundant rainfall and wind generators at night. Ammonia is seen as an excellent storage medium in this context since it can be stored economically for an indefinite period and can also be transported economically from the point of production to distant markets.
Battolyzer technology arouses excitement in this context because it is a single asset that can fulfill both short-term and long-term energy storage roles. Imagine a bank of battolyzers deployed at utility scale in association with renewable generating facilities. During normal periods – daytime, unexceptional weather conditions – the batteries charge and discharge according to signals received from grid authorities. During periods of sustained generation output at elevated levels, the excess electricity is used for electrolysis and subsequent ammonia production. The ability to generate two revenue streams from one asset promises to give the battolyzer an economic advantage over systems based on single-use assets.
Industry players are paying attention, in any case. The TU Delft Web post notes that Geert Laagland of the electricity utility Nuon envisages the technology playing a role at its cutting-edge Magnum power station (featured in previous Ammonia Energy posts). “If there is not enough wind and solar power, we can use the ammonia to supply clean power on a large scale immediately. Nuon is a member of the STW user group. We’re very curious about cost price, of course, but also flexibility. How quickly can you switch over?”
TU Delft reports that the Dutch technology transfer agency TTW (formerly STW) and several companies have come forward to fund a research program that can advance battolyzer technology.