Ammonia cracking to high-purity hydrogen, for PEM fuel cells in Denmark
By Trevor Brown on February 17, 2017
A start-up company in Denmark is commercializing technology to generate low-cost, high-purity hydrogen from ammonia, for use in fuel cells.
RenCat is a recent spin-off from the Technical University of Denmark (TU Denmark), and was founded by Ib Chorkendorff and Debasish Chakraborty. Keen readers will note that I wrote earlier this month about electrocatalysis research coming out of the same lab at TU Denmark, for renewable hydrogen generation and ammonia synthesis, but also that Chorkendorff was the only international researcher to participate in the US Department of Energy’s Roundtable on Sustainable Ammonia in February 2016.
The RenCat system claims to reduce the cost of the “cracking” process, which turns ammonia into hydrogen, by replacing the traditional Ruthenium-based catalyst with an Iron-Nickel alloy.
[Fe-Ni] on alumina egg-shell catalysts optimized for high efficiency ammonia cracking: Our test results shows that the catalysts performs better than the Ru/Al2O3 typically used for high conversion ammonia cracking while bringing the catalysts cost [down] significantly because both Fe and Ni are inexpensive metals compared to Ru which is a noble metal.
RenCat website, accessed 02/16/2017
But the heart of RenCat’s technology is a second innovation, “a mixed metal oxide catalyst which can remove trace ammonia impurity in hydrogen to the level of 0.1 ppm.”
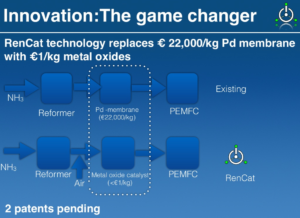
This allows RenCat’s system to turn ammonia into “fuel cell grade hydrogen” for use in a proton exchange membrane, or polymer electrolyte membrane fuel cell (PEMFC), at a competitive cost.
The process of cracking ammonia into hydrogen leaves trace amounts of ammonia in the fuel supply, which would otherwise poison a PEM fuel cell. According to RenCat, the existing technology for purifying this hydrogen stream requires a palladium catalyst, which is “expensive and unreliable … making H2 generation from ammonia unfeasible,” until now.
The RenCat system also has much milder operating conditions: Palladium-based systems can run at 600 °C and 10 bar, whereas RenCat’s metal oxide-based system runs at less than 300 °C and 1 bar. The lower temperature and ambient pressure also reduce the system’s costs.
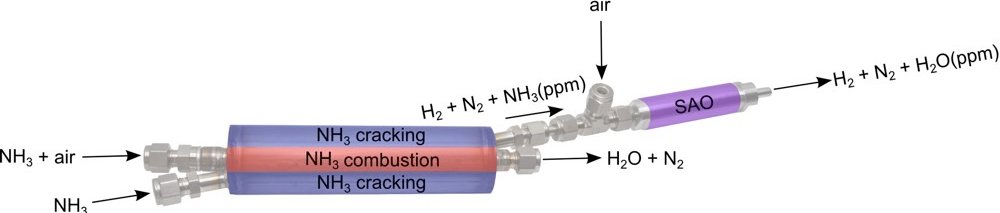
RenCat technology uses an inexpensive mixed metal oxide based catalyst to selectively oxidise the trace ammonia after the cracking step to generate a PEMFC quality H2. The patent pending catalyst brings ammonia concentration down to zero by selectively oxidising ammonia from the H2 containing stream via a process called selective ammonia oxidation (SAO) …
Because of the use of very inexpensive metal oxides and metal alloys as catalyst, the price of H2 generator will be at least an order of magnitude lower compared to Pd-membrane based system.
RenCat website, accessed 02/16/2017

RenCat is currently marketing both the Fe-Ni alloy catalyst and the complete ammonia-fueled hydrogen generation system, although for now it is only a small-scale version “suitable for education and testing purposes.”
This “bench top hydrogen generator,” called the RenGen, is sized to generate hydrogen streams of either 100 ml or 1 liter per minute.
The project has sparked significant interest already, starting with the support of local start-up accelerator Climate-KIC, which in 2014 awarded the project 20,000 EUR funding “to build a prototype of the generator, and … assistance in finding our first partners.”
The first partner was significant: GrameenPhone, “the biggest telecoms company in Bangladesh,” which entered into an MOU with RenCat in August 2015. According to an article on the TU Denmark website, “the plan going forward is to test RenCat generators in the field in partnership with Grameen Phone.”
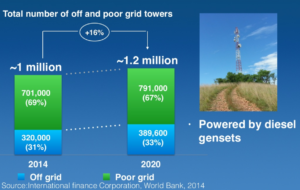
RenCat’s initial target market is stationary power in developing countries, where the electric grid either doesn’t exist or isn’t reliable – specifically, the fast-growth market for supplying power to remote cell phone towers across Africa and Asia. Grameen Phone currently operates roughly 11,000 cell phone towers across Bangladesh, serving 60 million customers.
The incumbent technology in this market is the diesel genset. By switching to an ammonia-fed PEM fuel cell, consuming ammonia produced from a natural gas or coal feedstock, RenCat claims to provide a 40% operating cost reduction, and a 30% reduction in carbon emissions (if the ammonia fuel were produced using renewable power instead of fossil fuels, the reduction in carbon emissions would be 100%).
According to RenCat, the team is now “in the process of integrating our ammonia to hydrogen generator with a commercially available fuel cell module.”
This month, they were awarded an additional 50,000 EUR through the EU’s Horizon2020 program, for “Onsite, On-demand, Self-standing Cost Competitive Zero-Carbon Power Generation”. This SME Phase-1 grant will fund the feasibility study and, once that is complete, “we plan to apply for the SME Phase-2 … to develop and commercialize the technology.”
RenCat is preparing to launch into adjacent markets, including other off-grid back-up power applications like military deployments, refugee camps, and hospitals. However, the team is also exploring more mainstream, mass market opportunities, with a specific interest in the potential to support carbon-free power generation in the future hydrogen economies that will rely upon imported ammonia. We’ve written previously about those vast potential markets, in Japan and in Germany, that could fuel their carbon-free electric grids using imported solar ammonia.