A road ahead via lithium-mediated electrochemical nitrogen reduction?
By Kevin Rouwenhorst on October 03, 2022
The direct synthesis of ammonia from air and water via electrochemistry has been a scientific challenge for several decades. However, various research groups have recently made advances with a new technique: lithium-mediated electrochemical nitrogen reduction for ammonia synthesis.
The promise of electrochemical nitrogen reduction
The ammonia synthesis reaction is an exothermic reaction, resulting in an energy loss from the system. The theoretical minimum energy consumption for electrolytic hydrogen production coupled with the Haber-Bosch process is 21.3 MJ per kg ammonia, while current commercial processes typically consume 36 MJ per kg ammonia. Totaled across global production units, this a massive energy consumption load.
However, if ammonia is directly synthesized from nitrogen and water via electrochemistry, the heat loss from the ammonia synthesis step can be avoided, decreasing the theoretical minimum energy consumption to 18.6 MJ per kg ammonia. Add the potential flexible load operation of electrochemistry to this, and it is not hard to see why many – if not all – electrochemistry research groups have tried to make this happen. However, it has proven to be a herculean scientific challenge.
Setting protocols & standards
Progress has been made over the past few years defining a rigorous protocol for experimental measurements, and the identification of false positives from contaminations. Broadly-accepted benchmarks have also been set regarding the reaction rates (>265 grams ammonia per square meter per hour) and Faradaic efficiency (>50%) required for commercialization.
So far, most of the reported ammonia synthesis rates are far below this target, at least two orders of magnitude lower than the estimate, and at Faradaic efficiencies only as high as 20%.
Lithium-mediated ammonia synthesis
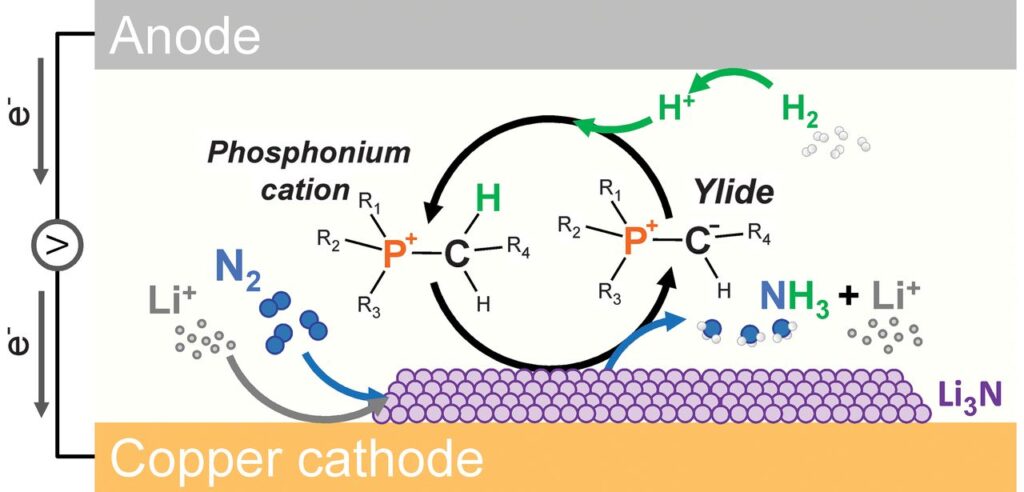
Recently, researchers have intensified their focus on Lithium-mediated ammonia synthesis, and to great avail. Last year, we reported that a team at Monash University in Australia managed to obtain a Faradaic efficiency of 69% for a 20-hour experiment, at a reaction rate of 32 grams ammonia per square meter per hour using “shuttling”. Further optimization of the electrolyte improved the Faradaic efficiency to nearly 100%, at a reaction rate of 92 grams ammonia per square meter (as reported this year in Nature).
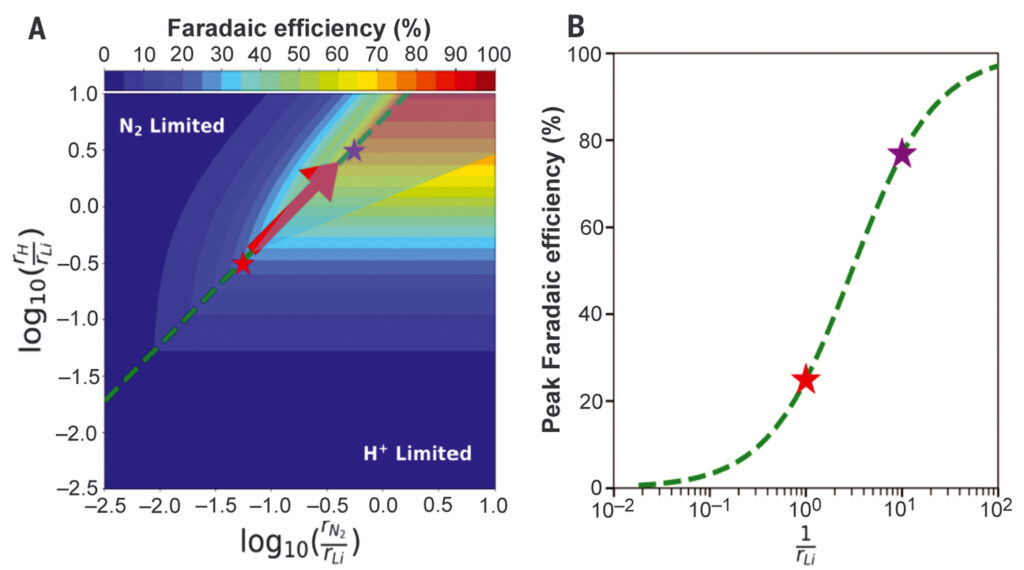
Researchers at the Technical University of Denmark (DTU) have also made advances to improve Lithium-mediated ammonia synthesis. By adding up to 1% oxygen, Faradaic efficiency in their system increased from 25% to 80%. The same group also recently reported production rates as high as 1533 grams ammonia per square meter per hour, at a Faradaic efficiency of 71%. Further improvements may be possible by engineering the electrodes.
In conclusion, the benchmark of at least 265 grams ammonia per square meter per hour at a Faradaic efficiency above 50% has been achieved in published literature This means the scientific challenge has been (partially) overcome. Now the next challenge begins: engineering an electrochemical ammonia synthesis process with adequate product separation and purification.