An Open Letter to the International Energy Agency
By Stephen H. Crolius on July 08, 2019
To the Authors of The Future of Hydrogen:
First I would like to thank you for an excellent report. I published Ammonia Energy articles devoted to The Future of Hydrogen on June 20 and June 27. If you read them, you will see that my appraisal is overwhelmingly positive. But I am writing this letter because I take issue with the following sentences from page 35 of your report:
“Ammonia generally raises more health and safety considerations than hydrogen, and its use would probably need to continue to be restricted to professionally trained operators. It is highly toxic, flammable, corrosive, and escapes from leaks in gaseous form.”
I hereby submit that this description of ammonia’s hazard profile is inaccurate and unhelpful. Many similar assertions from other parties can be found, but I have chosen to write to you because you represent one of the preeminent institutions in the global energy sector. As you go forward with your illumination of the opportunities and challenges of hydrogen energy, it is essential that you comprehend and depict ammonia’s hazardous characteristics in a complete and precise manner.
Toxicity
I will focus on your three dimensions of hazard: “toxic, flammable, corrosive.” In my experience, “toxic” is indeed the first word people think of in connection with ammonia. And, according to an on-line course from University College London, “ammonia is highly toxic.” Case closed? Not exactly. Ammonia is “produced in most cells of the body, as a result of deamination of amino acids and amines.” It becomes toxic when it exceeds a concentration in the blood of 50 micromoles per liter. The crux of the matter is the circumstances under which blood ammonia could rise beyond this threshold. For example, could it happen because people are exposed to an ammonia cloud in the course of an industrial accident?
The answer is no. The only way the threshold can be exceeded is through a breakdown in cellular function within the body. According to the on-line course, “ammonia intoxication occurs when blood ammonium rises because the capacity to detoxify it by formation of glutamate and glutamine has been exceeded. Sometimes [this occurs] in infants whose blood ammonium has risen dangerously high (commonly as a result of a genetic disease affecting amino acid metabolism) . . . sometimes in adults with liver failure.” It is physically impossible for ammonia intoxication to occur via exposure to an external source of ammonia.
Corrosiveness
Since, in the context of public safety, neither ammonia nor hydrogen is toxic, toxicity can not be the basis for differentiated hazard profiles. But this is definitely not the case for the “corrosive” dimension. In the description of the Occupational Safety and Health Administration (OSHA) in the United States, “ammonia is considered a high health hazard because it is corrosive to the skin, eyes, and lungs. Exposure to 300 parts per million (ppm) is immediately dangerous to life and health.” This is clear and serious: ammonia is an unequivocally hazardous substance.

However, the question of exposure requires deeper scrutiny. To be harmful, exposure must consist of physical contact at a high enough concentration over a long enough period of time. In the context of an accidental release, the key parameter is ammonia concentration at the air-tissue interface. OSHA says the human nose can detect ammonia at 20 ppm. This happens to be below OSHA’s “permissible exposure level” over an eight-hour period (time-weighted average) of 25 ppm. In a hypothetical release, people who are conscious will smell ammonia and move away from it with all the speed they can muster. One factor working in their favor is that ammonia is less dense than air and hence subject to spontaneous dispersal. Finally, ammonia does not act in the manner of a nerve agent, where a little bit of contact can overcome a human being. A little bit of contact with ammonia leads to a little bit of tissue irritation. A large impact can only come from a large amount of contact. This is not to say it is impossible for an ammonia release to cause human harm; just that there are a number of factors that tend to limit the probability and degree of harm. (This point receives real-world validation in an article we published in 2017, U.S. EPA’s Toxicological Review of Ammonia.)
Flammability
To summarize, ammonia is corrosive but not toxic, while hydrogen is neither toxic nor corrosive. But before drawing conclusions about the relative degree of health and safety risk, it is essential to consider your final dimension, flammability. Ammonia is certainly flammable. It is an attractive energy vector precisely because of this quality, like every other fuel. But, unlike every other fuel, flammability is not one of ammonia’s leading risk factors. Ammonia just does not burn with much intensity. Developers of ammonia combustion devices often mention its low flame speed, which is a fraction of that of hydrocarbon fuels. This no doubt figures into the decision by the United States’ National Fire Protection Association (NFPA) to assign ammonia a flammability rating of 1 on a scale of zero to four. On this scale, zero indicates a non-flammable material and four is reserved for substances such as methane, propane, and acetylene whose gaseous form at ambient pressure and temperature promotes rapid combustion. So yes, ammonia is flammable, but “highly flammable” it is not.
Hydrogen is different. Its “highly flammable” nature earns it a four from the NFPA. To bring the comparison into sharper focus, the authors of Safety assessment of ammonia as a transport fuel, a 2005 report by Denmark’s Risø National Laboratory, cite a metric called the “RF Index.” The RF Index is composed of two factors, the first of which is the ratio between a fuel’s upper and lower flammability limits. Ammonia has a lower flammability limit of 15%, meaning that an air-ammonia mixture must contain at least 15% ammonia by volume to burn when an ignition source is present. Ammonia’s upper flammability limit is 28%, meaning an air-ammonia mixture will not burn if the ammonia concentration is above this level. Hydrogen’s lower and upper flammability limits are 4% and 75%, respectively. Compared to hydrogen, therefore, ammonia has a high flammability threshold (i.e., a large amount must be present before it can ignite) and a narrow range of flammable concentrations (making ignition impossible in relatively short order after it reaches that threshold).
The second factor in the RF Index is the ratio between heat of combustion and the molecular mass of the fuel. Ammonia has a heat of combustion of 383 kJ per mole (higher heating value). Hydrogen’s heat of combustion is only 286 kJ per mole (HHV), but, at two grams per mole, its molecular mass is a small fraction of ammonia’s 17 grams per mole. The RF Index uses this ratio, and not the heat of combustion by itself, because a light molecule that packs a lot of energy will have more extreme combustion kinetics than a similarly endowed heavier molecule.
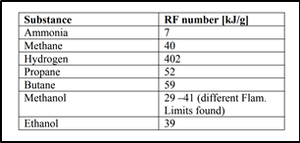
When the values above are plugged into the RF Index equation and the internal arithmetic is completed, ammonia emerges with an RF number of 7 and hydrogen with an RF number of 402. Based in part on this result, the Risø authors conclude that “ammonia is by far the less hazardous compound due to ignition probability and hazard. Hydrogen is by far the most hazardous substance.”
Implications
So given the points in the discussion above, should ammonia’s use “be restricted to professionally trained operators,” as you suggest? A determination on this point should be made in due course by qualified safety engineers engaged in the design of facilities that meet or exceed the acceptable risk levels defined by local regulators. But the relevant parties can and should examine the experience that is rapidly accruing for hydrogen, for example in the context of supplying energy for products powered by fuel cells. Here is OSHA’s discussion of the “job hazards” associated with hydrogen fuel cells:
Hydrogen used in the fuel cells is a very flammable gas and can cause fires and explosions if it is not handled properly. Hydrogen is a colorless, odorless, and tasteless gas. Natural gas and propane are also odorless, but a sulfur-containing (Mercaptan) odorant is added to these gases so that a leak can be detected . . . Hydrogen is a very light gas. There are no known odorants that can be added to hydrogen that are light enough to diffuse at the same rate as hydrogen . . . Hydrogen fires are invisible and if a worker believes that there is a hydrogen leak, it should always be presumed that a flame is present.
OSHA Web page: “Green Job Hazards: Hydrogen Fuel Cells – Fire and Explosion“
This sounds serious to my layman’s ear. Yet relevant authorities in numerous jurisdictions have determined that hydrogen is safe enough for fuel cell vehicle owners to conduct fueling operations without help from professionally trained operators. Given this setting of the “safe-enough” bar, it will be interesting to see how the safety engineers position ammonia in comparison.
My aspiration in writing this letter is to elevate the caliber of discourse on ammonia’s hazard profile. A century’s worth of knowledge exists on ammonia hazards and safety, including relative to its use as a fuel. (In addition to the Risø report, Quest Consultants prepared Comparative Quantitative Risk Analysis of Motor Gasoline, LPG, and Anhydrous Ammonia as an Automotive Fuel for Iowa State University in 2009. Its scope and methods are not identical to those of the Risø report, but its conclusions are similar.) It is imperative that we move beyond rote repetition that ammonia is “toxic” and into genuine engagement with the question of how its hazard profile can be managed with at least as much success as we have achieved with diesel fuel, gasoline, LPG, and CNG.
I would genuinely welcome a response to this letter.
Respectfully,
Steve Crolius
Co-Editor, Ammonia Energy