On the Ground in Germany
By Trevor Brown on February 09, 2017
Yet another national laboratory is developing technology for renewable ammonia, this time in Germany at the DLR, the German Aerospace Center (Deutsches Zentrum für Luft und Raumfahrt).
At the Institute of Thermodynamic Engineering (Institut für Technische Thermodynamik), the DLR is developing a method for electrochemical ammonia synthesis at ambient conditions.
This project aims to investigate an alternative route to produce ammonia without the need of hydrogen, high temperatures, high pressures and without CO2 emission … an idea developed at the DLR to synthetize ammonia via electricity in an electrochemical membrane reactor.
DLR, Master’s Thesis application: Electrochemical Ammonia Synthesis, February 2017
There’s not yet much information about this project (the link above is a job offer), which doesn’t appear to build upon published research, but it adds to a growing body of work already well underway at the DLR.
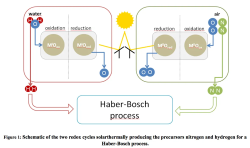
While these researchers bypass the need for a hydrogen feedstock, presumably using water as the hydrogen input, their colleagues in the department of Solar Chemical Engineering at the DLR’s Institute of Solar Research have been busy developing technologies for solar ammonia, solar hydrogen, and also solar nitrogen.
This latter is unusual because, while many new ammonia processes use electrolytic hydrogen from renewable power or derive hydrogen directly from a water feedstock in an electrochemical cell, as above, almost every new process relies upon existing air separation technology for the production of nitrogen.
At the DLR, however, they propose integrating nitrogen production within the concentrated solar power (CSP) system.
This technology for the solar production of nitrogen won the DLR’s biannual “Contest of Visions” in late 2014, and has been in steady development since then. “While scientists at DLR and elsewhere are already working on methods of solar hydrogen production, solar nitrogen production will break entirely new ground … The chances of the key step, the solar-thermal production of nitrogen, proving technically feasible are rated very highly by the visionaries at the Institute of Solar Research.”
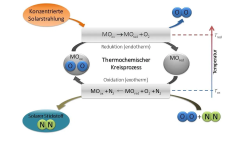
Thermochemical redox cycles enable the use of concentrated solar power or process heat for water splitting or air separation. The latter process is … achieved by oxidation of oxygen deficient oxides, which can be regenerated in a second step at high temperatures via thermal reduction …
Two promising candidate materials (SrFeOx and CaMnOx) have been identified, and their air separation performance was further optimized via doping … The observed reactions are completely reversible and the temperature window of application is in the range of 400‐1200 °C.
Their application for air separation processes appears highly promising and the feasibility of perovskite‐based air separation on the laboratory scale has been demonstrated.
DLR thesis: Development of Materials for Solar Thermal Production of Nitrogen as a Basis for Regenerative Fertilizer Production, March 2016
An associated research paper, published in August 2016, states that the project aims to develop materials “to pave the way for competitive air separation based on thermochemical cycling.”
The end application for this research, ammonia synthesis, requires the solar nitrogen process to tie in with a solar hydrogen process, both of which will feed into a traditional Haber-Bosch ammonia reactor.
This is not a technology for small-scale, distributed ammonia production, like so many I’ve described in other articles on renewable ammonia. Indeed, unlike other renewable power sources, such as wind or photovoltaic solar power, CSP is an inherently large-scale technology. As another DLR paper from November 2015 explains, the “concept opens up a novel research field that combines the upcoming technologies of solarthermal chemistry based on redox materials with industrial scale chemical processes.”
2017 may be an excellent year for the further development of this solar nitrogen-hydrogen technology: the DLR will, in March, dedicate the SynLight, “the largest artificial sun in the world.” This will provide researchers with experimental conditions at an industrial scale, for “the development of manufacturing processes for solar fuels” and other chemicals.
To illustrate the potential scale of this technology, which promises to integrate renewable power with industrial fuel and chemical production, the researchers created the following composite image of a solar-powered petrochemical complex, where renewable ammonia forms the basis for both fuel and chemical production.

You can also find the full article at AmmoniaIndustry.com.