Technology Status: Anion Exchange Membrane (AEM) Electrolysis
By Kevin Rouwenhorst on August 30, 2023
Alkaline electrolysis and Proton Exchange Membrane (PEM) electrolysis are already deployed at industrial scale for renewable ammonia production, and we are now seeing the first multi-MW applications for solid oxide electrolysis. For this week’s technology update, we investigate the “newest kid on the block”: Anion Exchange Membrane (AEM) electrolysis.
AEM: combining concepts from alkaline and PEM
The chemistry of AEM electrolyzers is the same as for alkaline electrolyzers – namely, hydroxide (OH–) ions are transported through the membrane. Also similar to alkaline electrolyzers, the reactions at the cathode and anode are catalyzed by nickel electrodes (although more expensive metals for AEM have been reported in literature). Nickel is substantially more abundant and cheaper than iridium and platinum, which are both used in PEM electrolyzers. Potassium hydroxide (KOH) concentrations in AEM electrolyzers can be substantially lower (down to 1 wt.%) than in alkaline electrolyzers (typically 20-30 wt.%).
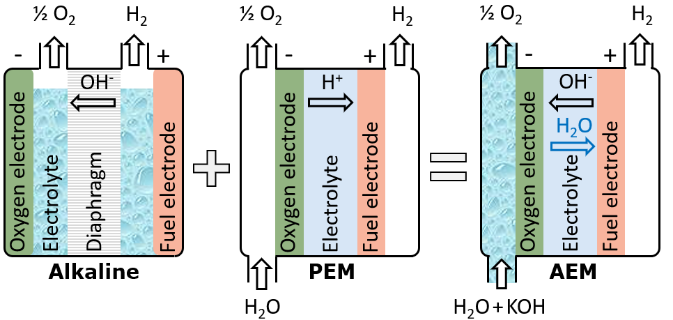
AEM electrolyzers benefit from the compact cell architecture like PEM electrolyzers. The highly acidic environment in PEM electrolyzers means that expensive titanium coatings are required on the bipolar plates. However, in case of AEM electrolyzers, nickel can be used instead. Due to the membrane between the cathode and anode, AEM electrolyzers can operate as flexibly as PEM electrolyzers without significant crossover of H2 and O2 (a potential safety concern with PEM electrolysis).
Although AEM electrolyzers can potentially offer “the best of both worlds” when compared to alkaline electrolyzers and PEM electrolyzers, significant technology development is still required for the technology to become competitive. For example, membrane lifetime is a key concern, as the current membranes are highly sensitive to oxygen. Scale-up of electrode cells from <300 cm2 currently to cell areas similar to those of PEM electrolyzers (1500 cm2) will be required to decrease the cost of manufacturing.
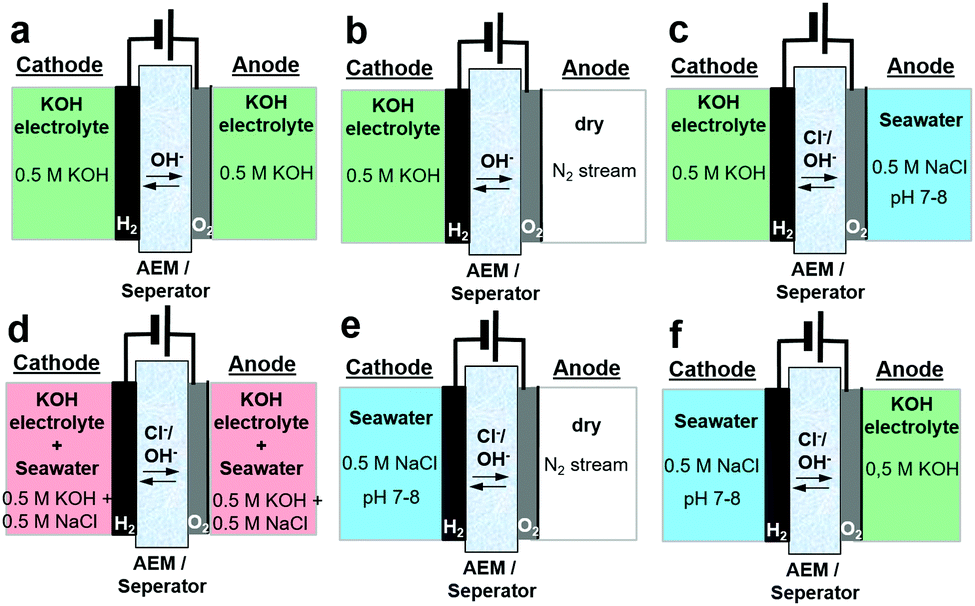
The current density is another important parameter, which scales inversely with the surface area. The commercially available AEM electrolyzer of Enapter has a current density of 0.2 A/cm2, compared to typical values of 1-2 A/cm2 for PEM electrolyzers. It should be noted that current densities up to 7.68 A/cm2 at 4.6 kWh/Nm3-H2 power input have been reported in literature for AEM electrolyzers with noble metal electrodes. Current densities of up to 1.62 A/cm2 have been reported without noble metal electrodes. Stable operation of AEM electrolyzers for over a 1000 hours has only been reported at much lower current densities of 0.5 A/cm2 and at 60°C. Radically novel concepts have also been researched, such as seawater-fed AEM electrolyzers, as reported by Dresp and coworkers.
Enapter: commercializing AEM electrolyzers
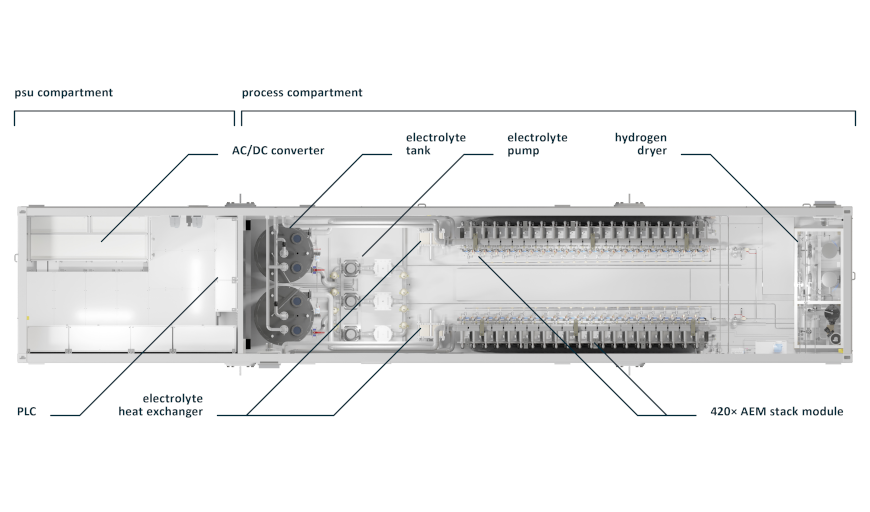
The first company to commercialize AEM electrolyzers is Enapter. Rather than scaling up the size of its stacks, Enapter has started off by mass-manufacturing 2.4 kW-sized systems based on relatively small stack units. Enapter’s “AEM Multicore” has 420 stacks (or “cores”) in a 1 MW system, producing about 20 kg hydrogen per hour.
Enapter has a manufacturing location in Saerbeck, Germany, where Enapter claims a production capacity of 10,000 electrolyzer systems per month. This translates into an electrolyzer manufacturing capacity of nearly 300 MW per annum.

Other companies are also developing and commercializing AEM electrolyzers, including Alchemr, EvolOH and HydroLite. Existing alkaline electrolyzer and solid oxide electrolyzer company Sunfire has also announced that it will develop AEM electrolyzers.