The Future is Here for Solid Oxide Electrolysis Cell Technology
By Stephen H. Crolius on October 22, 2020
Earlier this month the journal Science published “Recent advances in solid oxide cell technology for electrolysis.” Given this title, readers might reasonably expect the paper to describe research on, for example, the charge transfer reaction at the triple phase boundaries within the fuel electrode. It does do that, but it also advances two important theses: first, solid oxide electrolysis cell (SOEC) technology has an important role to play in the sustainable energy economy of the future; second, SOEC technology has achieved a set of economics that make commercial viability possible today.
The paper includes a description of the authors’ long history in SOEC development. Chemical engineering firm Haldor Topsoe has been working with researchers at the Technical University of Denmark on SOECs for more than ten years. (Authors also include representatives from Aalborg University and the Danish electric grid manager Energinet.) The challenges confronted by the group over this time are ticked off in the paper’s subheadings at both the electrolysis cell and stack levels. (Example of the former: in the aforementioned triple phase boundaries within the fuel electrode, “advances in imaging techniques have made it possible to quantify the concentration and three-dimensional (3D) distribution of TPBs in real electrodes, enabling further improvements in electrode performance.” Example of the latter: “substantial progress” has been made to improve the individual and collective durability of “metallic interconnects, glass sealings, and flow channels.”) The result is that “the initial electrochemical performance of state-of-the-art SOEC single cells has more than doubled, while long-term durability has been improved by a factor of ∼100. Similar improvements in performance and durability have been achieved on the stack level.”
Oct 2020.
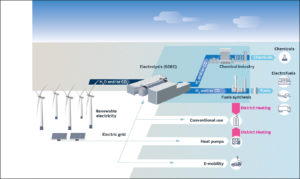
Having shared the technical news, the authors give equal time to drawing out its far-reaching implications. First, SOECs are uniquely qualified to fulfill a key role in a de-carbonized energy economy. One factor is their “unrivaled conversion efficiencies — a result of favorable thermodynamics and kinetics at higher operating temperatures.” Another is the ability to be “operated in reverse, [so that] an SOEC functions as a solid oxide fuel cell (SOFC),” i.e., after splitting water into hydrogen and oxygen, the flows can be reversed with stored hydrogen used as a fuel to generate electricity. A third is that SOECs stand up well to “dynamic load operation,” i.e., to conditions of fluctuating power levels, for example when following the output of wind-powered generation. A fourth is that an SOEC “can be thermally integrated with a range of chemical syntheses, enabling recycling of captured CO2 and H2O into synthetic natural gas or gasoline, methanol, or ammonia, resulting in further efficiency improvements compared with low-temperature electrolysis technologies.”
The dynamic load operation and integrated chemical synthesis capabilities are especially important in the context of a de-carbonized energy economy:
Electrolysis technologies will play a central role in future energy systems, acting as a vital link between the electric, gas, and thermal grids and providing fuel for the transportation sector … Studies show that greater than 40 to 50% penetration of wind power and photovoltaics in the electricity system requires further sector integration in combination with efficient energy conversion and storage technologies such as electrolysis … In general, gigawatt capacities installed on the production side need an aligned flexible demand. On a European scale, analyses show that 1600 gigawatt electrolysis and 7500 terawatt-hours of chemical storage may be needed to completely decarbonize heavy-duty transport such as trucks, ships, and planes.
Hauch, A., et al., Recent advances in solid oxide cell technology for electrolysis, Science
09 Oct 2020: Vol. 370, Issue 6513
The second essential implication is that SOECs, long a technology of the future, have become a commercial proposition of the almost-present. Indeed, “… multiple commercial (i.e., subsidy-free) plants with capacities of 96 Nm3/hour CO are to be commissioned in the next two years.” The authors go on to lay out their program for additional R&D at the cell, stack, and system levels. These improvements would augment the effects of the industrial scale-up that could cut production costs by almost three quarters by 2050.
Competitively advantaged capital costs could lead to massive deployments, and this brings another consideration into play: “The scale at which electrolysis cells will have to be deployed in future energy scenarios necessitates that any viable electrolysis technology be based on Earth-abundant materials.” SOECs make use of two compounds whose relative scarcity leads to high unit prices: zirconia, based on the element zirconium, and yttria, based on the element yttrium. They conclude that “solid oxide cells providing 1 TW of power in fuel cell mode would require just 1 month’s worth of global ZrO2 production and 21 months’ worth of Y2O3. To put these numbers into perspective: 24 hours of 1 TW of power generated using Li-ion batteries would require Li corresponding to ∼160 years of Li production in 2012, and 24 hours of 1 TW of power provided by a PEM fuel cell system would require 53 months’ worth of global Pt production.”
Last month Haldor Topsoe announced a major change to its internal organization. As described in the press release, the “new organization is designed to optimally drive the realization of the company’s vision to be recognized as the global leader in carbon emission reduction technologies by 2024.” Haldor Topsoe’s CEO, Roeland Baan, said that going forward the company will have “a clear focus on accelerating the development of carbon-neutral technologies.” The Science paper makes the case that SOECs will be one of those technologies.