Presentation
Electrochemical Nitrogen Reduction Reaction on Transition Metal Nitride Nanoparticles in Proton Exchange Membrane Electrolyzers
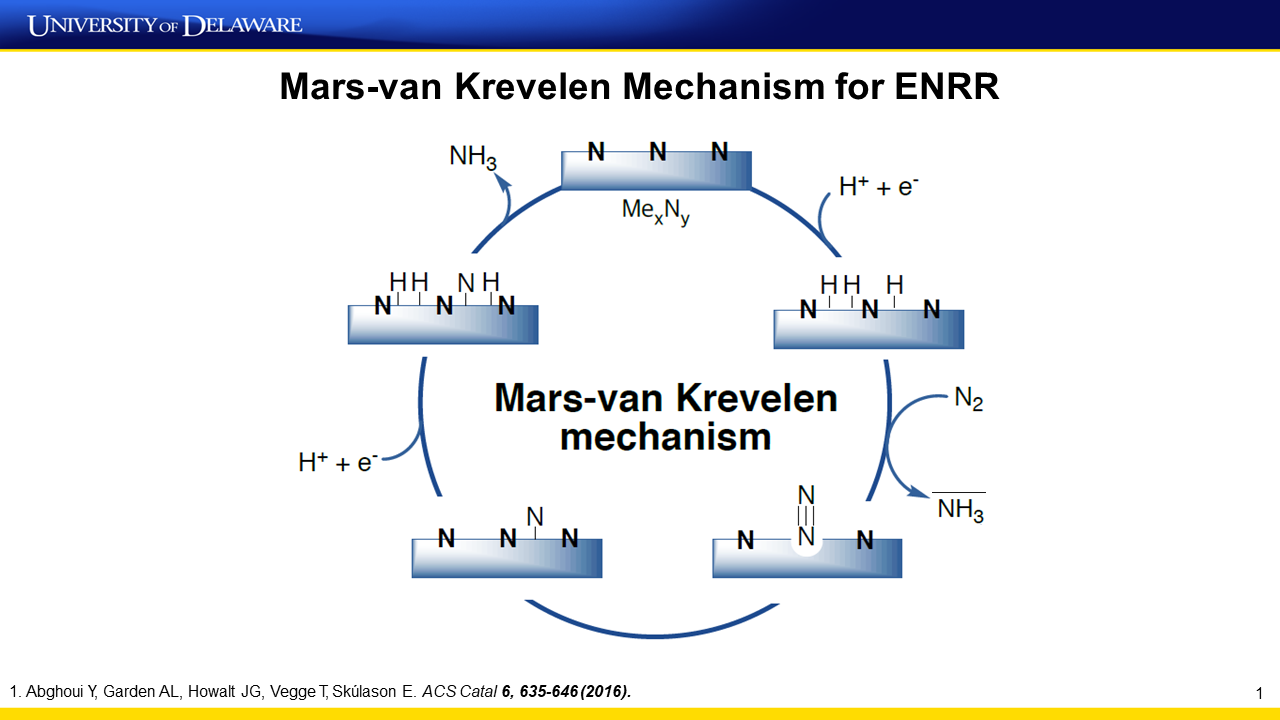
Transition metal nitride nanoparticles are synthesized and utilized as catalysts for electrochemical nitrogen reduction reaction (ENRR) to produce ammonia in a proton exchange membrane electrolyzer (PEMEL). The catalysts show an average ENRR rate and Faradaic efficiency (FE) of 3.3 × 10−10 mol s−1 cm−2 (6.6 × 10−10 mol s−1 mg−1) and 5.95% at −0.1 V within 1 h, respectively. Both the ENRR rate and FE are approximately two orders of magnitude higher than those of noble metal catalysts. Time-dependent results suggest that the catalytic activity of transition metal nitride nanoparticles is stable at −0.1 V, with the catalytic activity decreasing…