Presentation
Mechanistic Insights into Electrochemical Nitrogen Reduction Reaction on Vanadium Nitride Nanoparticles
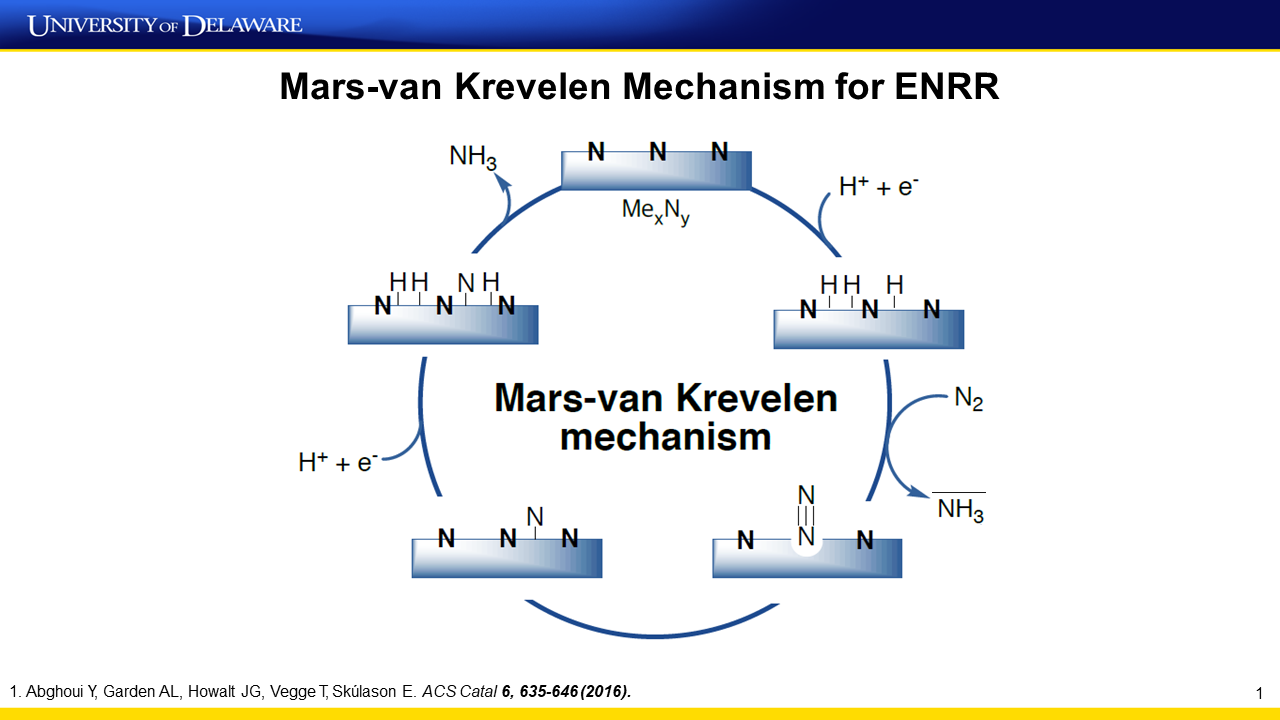
Renewable production of ammonia, a building block for most fertilizers, via the electrochemical nitrogen reduction reaction (ENRR) is desirable; however, a selective electrocatalyst is lacking. Here we show that vanadium nitride (VN) nanoparticles are active, selective, and stable ENRR catalysts. ENRR with 15N2 as the feed produces both 14NH3 and 15NH3, which indicates that the reaction follows a Mars–van Krevelen mechanism. Ex situ and operando characterizations indicate that VN0.7O0.45 is the active phase for ENRR and the conversion of VN0.7O0.45 to the VN phase leads to catalyst deactivation. Quantitative isotopic labeling results identify the amounts of two different types of…