NH3 vs. MCH: Energy Efficiency of Hydrogen Carriers Compared
By Stephen H. Crolius on May 09, 2019
Volume 174 of the journal Energy, published on May 1, 2019, includes a paper by Shin’ya Obara, Professor in the Department of Electrical and Electronic Engineering at the Kitami Institute of Technology in Japan, that should be of interest to hydrogen advocates everywhere. The paper, Energy and exergy flows of a hydrogen supply chain with truck transportation of ammonia or methyl cyclohexane, concludes that a hydrogen supply chain based on ammonia has better energy efficiency than one based on methyl cyclohexane (MCH).
Ammonia and MCH are two of the three hydrogen vectors that were studied by the Energy Carriers initiative within the Government of Japan’s Strategic Innovation Promotion Program between 2014 and the first quarter of 2019. (Liquid hydrogen is the third.) The Energy Carriers initiative was not intended to “pick a winner” from amongst its three study targets, but rather to illuminate the case and the path forward for each. And while there is no universal law of business that says multiple means of accomplishing the same end cannot coexist in a market economy, a typical outcome for competing options is for one to establish itself as the dominant solution – with lower cost often playing the decisive role in shaping the outcome. Obara’s paper is focused on energy efficiency, but it arrives at a conclusion that can only help ammonia establish a cost advantage over MCH.
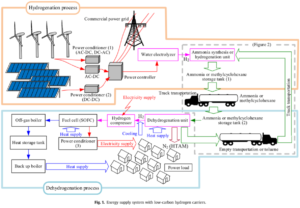
The paper lays out Obara’s analytic methodology in consummate detail, covering pertinent chemical reactions, energy flows at each process step, and macro system design. The design starts with the production of hydrogen by electrolysis at a location close to a solar or wind farm. In ammonia’s case, synthesis is via the Haber-Bosch process. In MCH’s case, the organic chemical toluene is used as the base commodity which is then reacted with hydrogen to produce MCH. After the initial hydrogenation step, the energy carriers are transported by truck to an integrated facility “near a region where demand has outstripped the load capacity of transmission lines.” Dehydrogenation ensues, followed by the conversion of hydrogen back into electricity in a solid oxide fuel cell (SOFC). For MCH, the byproduct toluene is returned to the hydrogenation plant for reuse.
One point of interest in Obara’s conclusions is the absolute value of “total energy efficiency” for the hydrogen carriers. Calculated for the electricity-to-electricity round trip, it is in the ballpark of 20 percent. This compares with values in the 30 percents for run-of-the-mill thermal generating plants. (This comparison is not apples-to-apples for a variety of reasons, some of which cut one way and some the other, but it is broadly indicative.) This is clearly a major disadvantage for hydrogen energy, but it does not cast the hydrogen alternative as an obviously non-viable proposition.
The paper’s marquee finding is the relative energy efficiency values of the two hydrogen carriers: 22.5 percent for ammonia; 18.0 percent for MCH. The fact that these numbers are not very far apart on the scale of 0 to 100 percent disguises the practical implication: if a given quantity of hydrogen moves through the system in the form of ammonia and generates 100 kWh, it will generate only 80 kWh if it moves in the form of MCH. This could clearly contribute to major cost advantage for ammonia.
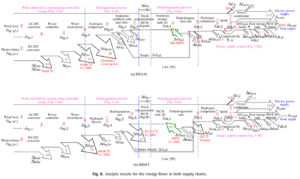
The story behind these bottom-line numbers is no less interesting. Producing ammonia consumes approximately 25 percent more energy per unit of hydrogen than the production of MCH. The former involves, among other things, a large energy investment in compression of hydrogen before it enters the Haber-Bosch synthesis unit. The process of hydrogenating toluene, by contrast, is done at low pressure and temperature. However, MCH gives up all of this advantage and more during the dehydrogenation step. The key factor is management of process heat. Dehydrogenation is conducted at moderately high temperature for both processes, but while this heat is effectively retained in the ammonia process (where the primary byproduct is gaseous nitrogen), it is not in MCH process, where gaseous toluene must be returned to liquid state via a process that does not lend itself as well to heat retention.
Obara’s rigor inspires confidence in the advantage shown for ammonia. But moving outside the parameters of the paper’s supply chain scenario in two important ways could increase the advantage significantly. The first relates to the physical distance between the hydrogenation and dehydrogenation plants. Obara sets this distance at one kilometer for the purposes of his analysis. This shrinks the difference in transport cost between ammonia and MCH to almost nothing. Yet in the real world, the distance is apt to be hundreds or even thousands of kilometers. This is problematic for MCH when its base commodity (toluene) must be returned to the hydrogenation plant after it has been dehydrogenated. Obara acknowledges this fact, stating in his conclusions, “For distances less than 40 km, the effect on energy losses is relatively small, but transportation fuel significantly affects system efficiency at greater distances.”
The second relevant parameter is the dehydrogenation step. Direct ammonia SOFC technology is well established and is marching toward commercialization. As Ammonia Energy reported in a May 2018 article, Direct Ammonia Fuel Cells Take Another Step Forward in Japan, the Japanese manufacturing concern IHI is one of the leaders in this field. Eliminating the dehydrogenation step in Obara’s ammonia supply chain would eliminate approximately a quarter of the energy losses in the system.
No one should jump to conclusions about how the race between ammonia and MCH will turn out. Many factors beyond energy efficiency will affect their ultimate cost competitiveness. But the results of Obara’s analysis certainly seem to place ammonia is on a promising track.