Sustainable ammonia synthesis: SUNCAT’s lithium-cycling strategy
By Trevor Brown on July 21, 2017
New research coming out of Stanford University suggests a fascinating new direction for renewable ammonia synthesis technology development.
The US-Danish team of scientists at SUNCAT, tasked with finding new catalysts for electrochemical ammonia production, saw that ‘selectivity’ posed a tremendous challenge – in other words, most of the energy used by renewable ammonia production systems went into making hydrogen instead of making ammonia.
The new SUNCAT solution does not overcome this selectivity challenge. It doesn’t even try. Instead, these researchers have avoided the problem completely.
The SUNCAT Center for Interface Science and Catalysis is a partnership between Stanford’s Engineering School and the SLAC National Accelerator Laboratory. It “explores challenges associated with the atomic-scale design of catalysts for chemical transformations of interest for energy conversion and storage.” I wrote about it back in February 2017, introducing its research towards “sustainable, fossil-free pathways to produce fuels and chemicals of global importance.”
SUNCAT works alongside the Technical University of Denmark’s (DTU) VILLUM Center for the Science of Sustainable Fuels and Chemicals, led by Ib Chorkendorff. An electrochemical ammonia production expert, Chorkendorff contributed to the US Department of Energy’s 2016 Roundtable Report, Grand Challenges in Sustainable Ammonia Synthesis, and has also developed other technologies to enable the broader ammonia economy. This includes RenCat, a spin-off from the university that aims to commercialize a technology to ‘crack’ ammonia back into high-purity hydrogen.
The ammonia synthesis research at both SUNCAT and DTU is funded through the VILLUM Center with the aim of solving the ‘grand challenge’ of developing the new, better catalysts needed for sustainable process technology.
Catalysts – compounds that spur reactions without being consumed – have been used on an industrial scale for more than a century. Today’s fertilizers are commonly derived from petrochemicals through an energy-intensive process that relies on catalysts to accelerate reactions that occur under high pressures and temperatures. Developing a low-energy, solar-based process to make nitrogen fertilizers could benefit billions of people, particularly those in the developing world. But to get there SUNCAT researchers will have to break ground in the science of catalysis.
“We know of no manmade catalysts that can do what we require,” Norskov says. “We will have to design them.”
Stanford news, Can we use solar energy to make fertilizer right on the farm?, 22/03/2017
Among the many papers published by this team since they started work is an excellent study of what makes electrochemical ammonia synthesis so difficult, and what poses the greatest challenge to sustainable ammonia catalyst design:
It is notoriously difficult to electrochemically reduce N2 at ambient conditions … From a thermodynamic perspective, electrochemical reduction is possible, but most attempts primarily produce hydrogen (H2) and very little NH3.
SUNCAT, Strategies to Improve Selectivity in Electrochemical Ammonia Synthesis, 02/21/2017
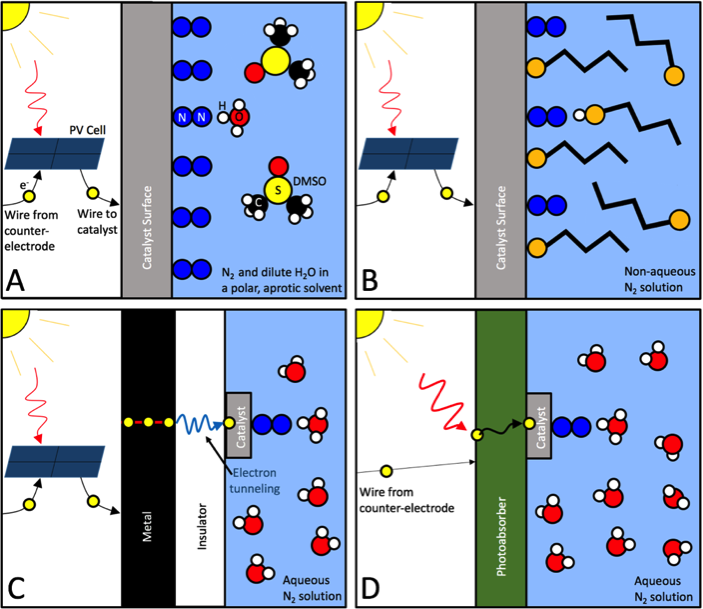
This is the ‘selectivity’ challenge, which every electrochemical ammonia synthesis technology yet measured has found daunting (see, for instance, my article from last month about one of the most promising technologies, from South Korea).
The conclusion of SUNCAT’s January 2017 paper, Electrochemical Ammonia Synthesis — The Selectivity Challenge, was that four strategies were available to overcome this challenge. Sadly, none were perfect: they all added system complexity and achieved greater selectivity by limiting the rates of proton or electron transfer.
Now, however, instead of designing a new catalyst for ammonia synthesis, SUNCAT’s latest research defines a new strategy for ammonia synthesis.
Instead of requiring the catalyst to have good selectivity, this new approach splits the ammonia synthesis reaction into a series of three separate reactions, each a phase in a cycle of reactions using lithium. With each phase of the cycle occurring separately (‘stepwise’), the selectivity question becomes moot.
Whereas traditional aqueous electrochemical approaches are typically dominated by the hydrogen evolution reaction (HER), we are able to circumvent the HER by using a stepwise approach which separates the reduction of N2 from subsequent protonation to NH3, thus our synthesis method is predominantly selective for ammonia production.
Nørskov et al, Ammonia synthesis from N2 and H2O using a lithium cycling electrification strategy at atmospheric pressure, Energy & Environmental Science, July 2017
This lithium cycle has three separate stages.
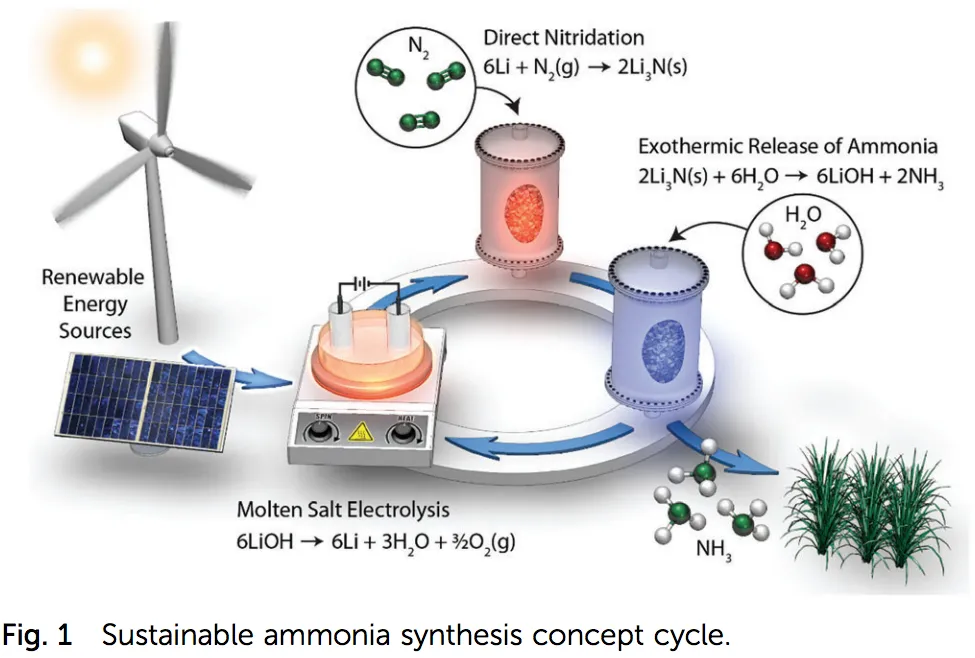
First, electrolysis of LiOH (molten salt) produces lithium (Li). Second, direct nitridation of lithium produces Li3N. And third, the exothermic release of ammonia from Li3N produces NH3 and LiOH, which – once the NH3 is removed – completes the cycle.
Each stage of this process takes place at atmospheric pressure and “reasonable” temperature, using inputs of just nitrogen (N2) and water (H2O).
Crucially, the results are orders of magnitude more efficient than other electrochemical ammonia technologies: “while approaching industrial level electrolytic current densities, we report an initial current efficiency of 88.5% toward ammonia production.”
“The fact that lithium binds nitrogen so strongly simply means that you pull down the barrier for nitrogen dissociation enough that you can do it at room temperature. So if you can make lithium, then you can also make lithium nitride …
“The trick in the process is to not do this in an environment where there is hydrogen present.”
Jens Nørskov quoted in Chemistry World: Lithium could hold key to sustainable ammonia synthesis, 07/18/2017
More research and ongoing funding will be required to move the technology forward. Nonetheless, this latest paper presents a road-map for development:
Theoretical analysis suggests that, based on this generalized strategy, other metal systems may continue to improve upon the metrics of efficient electrochemical ammonia production, opening up a new avenue of research to explore. While we have depicted a step-wise reaction scheme to effectively introduce and demonstrate the lithium reaction cycle, a continuous process in a compartmentalized device would be beneficial for implementation. Initial techno-economic electricity cost analysis and energy input considerations for this process reveal promise for suitable markets, especially considering the advantages of this process which can use renewable resources, mitigate CO2 emissions, and be readily de-centralized compared to conventional, centralized ammonia synthesis.
Nørskov et al, Ammonia synthesis from N2 and H2O using a lithium cycling electrification strategy at atmospheric pressure, Energy & Environmental Science, July 2017
You can also read the full article at AmmoniaIndustry.com.