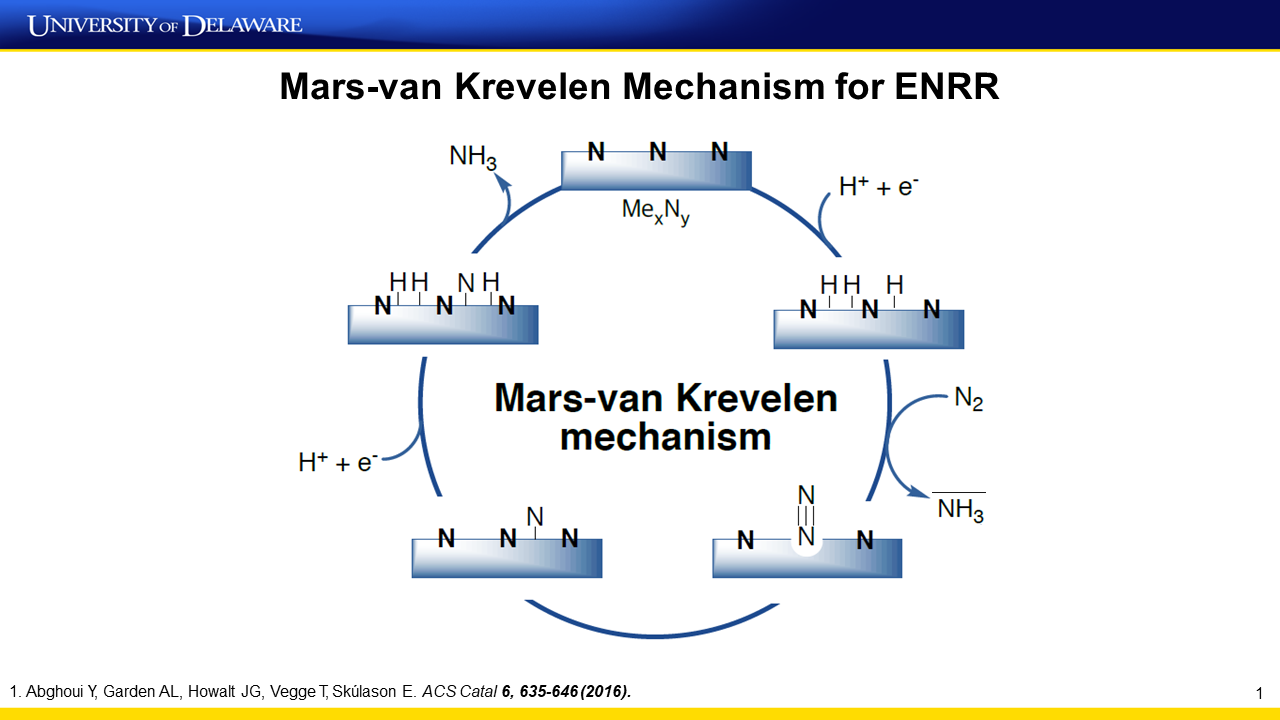
Presentation
Low-Pressure Electrolytic Ammonia Synthesis Via High-Temperature Polymer-Based Proton Exchange Membrane
The University of North Dakota Energy and Environmental Research Center (EERC) and North Dakota State University (NDSU) have developed a low-pressure electrolytic ammonia (LPEA) production process. The LPEA process uses an electrochemical cell based on an innovative polymer–inorganic composite (PIC) high-temperature (300°C) gas-impermeable proton-exchange membrane conceptualized and partially developed by EERC and NDSU. Because of its operability at ambient pressure and quick start-up capability (versus traditional high-pressure Haber Bosch-based plants), the LPEA process offers compatibility with smaller-scale plants and intermittent operation, and a cost-effective means of monetizing (and storing) renewable energy as ammonia. EERC, NDSU, and Proton OnSite are embarking…