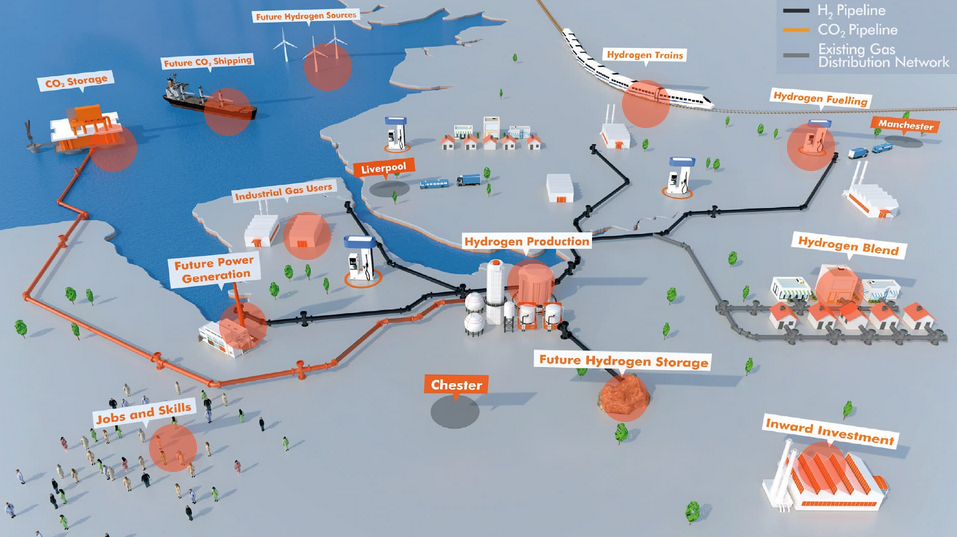
The Ammonia Academic Wrap: "seamless" cracking, improving Haber Bosch, a novel green power-to-ammonia-to-power solution and a review into the use of ammonia as a fuel
Welcome to the Ammonia Academic Wrap: a summary of all the latest papers, developments and emerging trends in the world of ammonia energy R&D. This week: "seamless" ammonia cracking tech from Northwestern, a new electrolysis catalyst, successful integration of ammonia synthesis and separation for improved efficiency, more research needed into transition metal catalysts for Haber Bosch, a novel, green power-to-ammonia to power system and a review on ammonia as a potential fuel.