The Ammonia Economy at the ACS National Meeting
By Trevor Brown on July 06, 2017
The American Chemical Society (ACS) has published the program for its 2017 National Meeting, which takes place next month in Washington DC and includes a session dedicated to the “Ammonia Economy.”
The first day of the week-long meeting, Sunday August 20th, will feature a full morning of technical papers from the US, UK, and Japan, covering ammonia energy topics across three general areas: producing hydrogen from ammonia, developing new catalysts for ammonia synthesis and oxidation, and storing ammonia in solid chemical form. The program for the Ammonia Economy session is available on the ACS website.
Hydrogen generation from ammonia
The first three papers focus on using ammonia to deliver hydrogen to the customer, enabling the safe and economic storage, transportation, and distribution of hydrogen fuel.
Ammonia has a high volumetric and gravimetric hydrogen density, which means that a large amount of energy is contained per volume and weight of fuel (per gallon or per kilogram). Hydrogen energy can therefore be stored and distributed safely and efficiently in the form of ammonia until, at the point of use, the ammonia is decomposed to generate high-purity hydrogen.
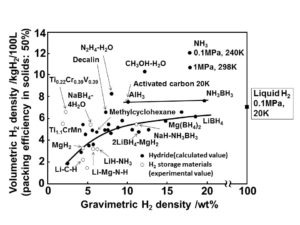
As Yoshitsugu Kojima from Hiroshima University explains, “Ammonia (NH3) is easily liquefied by compression at 1 MPa and 25°C, and has [the] highest volumetric hydrogen density of 10.7 kg H2 /100L,” and therefore “NH3 has advantages as a hydrogen carrier for fuel cell vehicles.”
The presentations cover research from Japan that aims to provide the technology necessary to distribute hydrogen at fueling stations for fuel cell vehicles – this project has been the subject of previous articles at Ammonia Energy. While the Japanese paper uses a “ruthenium supported on MgO catalyst,” two papers from the UK will detail progress in developing catalysts based on light metal amides and imides. These include lithium imide (Li2NH) and lithium-calcium imide (Li2Ca(NH)2), which “show high catalytic activity comparable to ruthenium-based catalysts, and thus present a low cost method of producing hydrogen from ammonia for utilization in energy storage applications.”
Catalysts for ammonia synthesis and oxidation
A joint project between Tokyo Institute of Technology in Japan and Pacific Northwest National Laboratory in the US is developing a synthesis technology focused on mild operating conditions.
Search for an efficient and stable catalyst enabling ammonia synthesis under mild conditions is a major topic in catalysis research. Recent experimental studies demonstrated that flat Ru nanoparticles on calcium amide [Ca(NH2)2] enable continuous synthesis of NH3 at temperatures as low as ~200 °C and ambient pressure. In order to extend these results to a broader class of catalysts, we aim to understand the atomistic origin of this low-temperature activity and to reveal effects specific to the supporting surface and the size and shape of Ru nanoparticles.
Phuong-Vu Ong et al, Structure and activation of Ru catalyst on Ca(NH2)2, ACS National Meeting, August 2017
At the opposite end of the ammonia energy value chain, researchers from Georgetown University will present their work on using ammonia as a direct fuel (ammonia oxidation), employing “copper-based molecular electrocatalysts … to convert NH3 to N2, protons, and electrons for ultimate use in ammonia fuel cells.”
Storing ammonia as a solid
Finally, two papers from the US and the UK address the emerging topic of storing ammonia in a solid chemical form, in “ammonium salts and metal amine chlorides,” or “metal organic framework materials.”
While this approach might appear to lower the efficiency and increase the weight of a system designed to deliver hydrogen energy, there are nonetheless important commercial opportunities for this technology – especially where safety is paramount and storing pure anhydrous ammonia might present unacceptable risks.
It is well known that ammonia is an active molecule for the selective catalytic reduction of NOx in the exhaust … Current technology employs the use of Urea dissolved in water or Diesel Exhaust Fluid (DEF) as ammonia source. However, this system has its own limitations such as freezing at low temperature (-12 degree C), solid deposit formation in the exhaust line, and difficulties in dosing ammonia at exhaust temperatures below 200 degree C. Another major drawback of [DEF] is its limited ammonia capacity, due to which there has to be an additional automotive fluid distribution system at every gas station …
Metal amines are very attractive functional materials due to their remarkable reversible ammonia storage property and a high volumetric ammonia density of up to 93% of that of liquid ammonia.
Abhijeet Karkamkar, Alternative ammonia storage materials for SCR of NOx, ACS National Meeting, August 2017
Similar technologies are already commercial and competing with DEF in the de-NOx market. For example, just last week the Danish start-up Amminex announced a new contract to install and test similar technology in London buses. Beyond the de-NOx market, however, the ability to transform hazardous anhydrous ammonia into a safe and stable solid form could open new energy applications and ultimately have a significant impact in niche clean energy markets.
All the abstracts for the Ammonia Economy session are available on the ACS website.