New Ammonia-Reforming Catalyst System
By Stephen H. Crolius on May 05, 2017
On April 27 the on-line journal Science Advances published “Carbon-free H2 production from ammonia triggered at room temperature with an acidic RuO2/γ-Al2O3 catalyst”. The lead author, Katsutoshi Nagaoka, and his six co-authors are associated with the Department of Applied Chemistry at Oita University in Japan. The innovation featured in the paper could prove to be an important enabler of ammonia fuel in automotive applications.
The Oita University team set out to address a specific challenge. As they wrote in the paper’s introduction, “Adoption of ammonia as a hydrogen source, especially for transportable devices and automobiles, has been limited, largely because of the absence of an efficient process for decomposing ammonia to hydrogen and nitrogen. Overcoming this issue will require the development of a process with rapid initiation and a high rate of hydrogen formation but without the need for external energy input.”
The innovation developed by Nagaoka and his colleagues involves a nanoparticle ruthenium oxide (RuO2) catalyst structured on a gamma-alumina (γ-Al2O3) substrate. The key feature is that “immediately after exposure of ammonia and O2 to [the] catalyst . . . at room temperature (~25°C), exothermic oxidative decomposition of ammonia is triggered, and hydrogen is produced at a high rate.” In other words, a device based on this catalyst could remain at rest for an indefinite period and then start to function immediately and spontaneously as soon as ammonia and oxygen are present. Initial testing indicates that the catalyst will maintain its functionality over many use cycles.
The paper does not identify specific applications for the technology within the transportation sector. The authors make clear, however, that the unique value delivered by the technology resides in its cold-start capability. This is entirely distinct from two other ammonia reforming technologies that were discussed previously in Ammonia Energy News. In both of these cases, the explicit target was an ammonia reforming system that could be used in hydrogen refueling stations. In July of 2016 a group at Hiroshima University reported that they had developed an ammonia reforming system that could deliver hydrogen that meets the standard set by ISO 14687-2 for fuel-cell vehicle (FCV) hydrogen, which specifies at least 99.97% purity and ammonia contamination of less than 0.1 parts per million. In February 2017, a group at Gifu University reported development of an alternative method that could deliver hydrogen with 99.999% purity.
The Oita University technology, by contrast, is intended for partial reforming only, with a maximum hydrogen conversion rate from NH3 to H2 of 67%. It thus a poor fit for hydrogen fueling but foreseeably an excellent fit for cars that take on ammonia fuel and burn it in internal combustion engines — and that need the ability to undergo frequent on-off cycles.
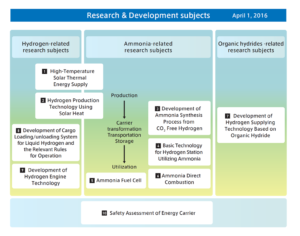
This is notable because ammonia-powered internal combustion engine (A-ICE) technology appears to be have attracted little notice within Japan’s program to develop a “hydrogen society.” A-ICE is included as a subtopic within the “ammonia direct combustion” element of the Cross-Ministerial Strategic Innovation Promotion Program (SIP)’s Energy Carriers research and development agenda, but it has been almost invisible in comparison with the “gas turbine power generation” subtopic under the same element. The latter has attracted considerable attention because of the success of the gas turbine program at Tohoku University. (It was the subject of an Ammonia Energy News story in February.)
At the same time, Japan’s leading automotive OEMs appear to have put the bulk of their “hydrogen society” efforts into fuel cell vehicles (FCVs). Although Toyota sought and received a series of patents earlier in the decade for A-ICE-oriented inventions, the company appears to be have placed strong priority on FCV commercialization leading up to and since the introduction of the Mirai at the end of 2014.
This means that the Oita University technology may find more interest in countries outside Japan. As reported previously in Ammonia Energy News, a number of groups are working on systems that utilize ammonia as an internal combustion fuel. They hail from the U.S., Canada, Italy, China, and South Korea. While some are thinking in terms of dual-fuel systems with a carbon-based “combustion promoter,” others are working on ammonia-only systems that will employ some form of ammonia-to-hydrogen reforming. For these groups, the Oita University technology could prove to be a critical part of the solution.
Last fall Nagaoka and colleagues from Oita University, Kyoto University, and Kyushu University, published “A low-crystalline ruthenium nano-layer supported on praseodymium oxidegifu as an active catalyst for ammonia synthesis” in the journal Chemical Science.