Electrochemical ammonia synthesis in South Korea
By Trevor Brown on June 09, 2017
One of the many encouraging announcements at the recent Power-to-Ammonia conference in Rotterdam was the news that the Korea Institute of Energy Research (KIER) has extended funding for its electrochemical ammonia synthesis research program by another three years, pushing the project forward through 2019.
The basic premise of the electrochemical approach is to produce ammonia directly from atmospheric nitrogen (N2) and water (H2O) using electricity in an electrolytic cell (which is akin to a fuel cell in reverse). While many variations of this technology have been demonstrated in recent decades, none have produced enough ammonia to be commercial (yet).
The KIER team has been working on electrochemical ammonia synthesis for at least five years already, and presented results at the annual NH3 Fuel Conference since 2012.

Now, KIER’s research target for 2019 is significant: to demonstrate an ammonia production rate of 1×10-7 mol/s·cm2.
If the KIER team can hit this target, not only would it be ten thousand times better than their 2012 results but, according to the numbers I’ll provide below, it would be the closest an electrochemical ammonia synthesis technology has come to being commercially competitive.
KIER’s research and development has evolved significantly since the program began. Back in 2012, it focused on solid state ammonia synthesis (SSAS), using a solid oxide electrolysis cell. In 2014, it moved away from solid electrolytes and began using using molten salt electrolytes (MSAS).
Now, according to Chung-Yul Yoo’s presentation at the Power-to-Ammonia conference in May 2017, Progress and Perspectives of Sustainable Ammonia Synthesis, KIER’s team, which also includes Hyung Chul Yoon and Jong-Nam Kim, has embarked on a new phase of research: liquid state ammonia synthesis (LSAS).
Molten Salt Ammonia Synthesis (MSAS)
The molten salt electrolyte used in KIER’s MSAS system was a “Eutectic mixture LiCl-KCl-CsCl in Ar atmosphere at 400 °C,” using an iron oxide nanocatalyst. (“Eutectic” describes a mixture of substances that melts at a lower temperature than each of its individual parts.) Using this system, KIER achieved an ammonia synthesis rate greater than 5 x 10-9 mol/s·cm2.
For those unfamiliar with performance measurement of electrochemical ammonia synthesis technologies, production is generally expressed in units of molecules per area per time. The area (cm2) could be considered the cost-driving factor, especially if the technology requires an expensive catalyst. Relative to traditional catalysts, nanocatalysts have a much greater surface area and so greatly improve the performance.
For reference, Giddey et al from CSIRO in Australia published an influential 2013 paper in the International Journal of Hydrogen Energy, Review of electrochemical ammonia production technologies and materials. According to their assessment, electrochemical systems had so far produced ammonia “in the 10-13–10-8 mol cm−2 s−1 range.” (KIER’s MSAS system fits in this range.)
The Giddey paper identified areas for further research and development, namely “electrolytes, catalysts and technology up-scaling,” and established a target production rate that its authors believed new electrochemical technologies would need to meet or exceed in order to be competitive with existing technologies.
Grover Coors described this rate as the “Giddey Commercial Benchmark” in his 2016 NH3 Fuel Conference presentation, Progress in the Electrochemical Synthesis of Ammonia, in which he presented research updates from Stoukides et al at CERTH and Aristotle University in Greece. Coors expressed the Giddey Commercial Benchmark as an ammonia production rate greater than 1 x 10-6 mol/s·cm2, at >50% Faradaic Efficiency.
Liquid State Ammonia Synthesis (LSAS)
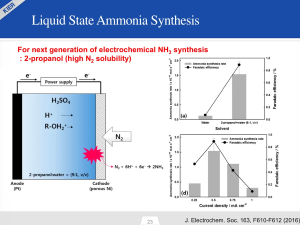
In a preview of early results, Yoo introduced KIER’s LSAS technology as a potential “next generation of electrochemical NH3 synthesis.”
The liquid electrolyte that KIER uses in its LSAS technology is 2-propanol, an alcohol selected for its high nitrogen solubility.
As I mentioned above, KIER’s stated target for 2019 is an ammonia production rate of 1×10-7 mol/s·cm2. This would be an order of magnitude greater than its own 2016 results as well as all the other technologies assessed in the Giddey paper, and (only) one order of magnitude away from meeting Giddey’s Commercial Benchmark. This would be a significant achievement.
According to a statement by Chung-Yul Yoo:
In March 2017, KIER (Korea Institute of Energy Research) launched the second phase of the project “CO2-free ammonia synthesis” in cooperation with Korea Advanced Institute of Science and Technology and Chung-Nam National University. The second phase of the project focuses on the electrochemical ammonia synthesis together with concentrating dilute ammonia in the effluent stream.

Readers with long memories may remember that research coming out of KIER was the subject of one of my first articles about ammonia, four years ago, on The AmVeh – an ammonia fueled car from South Korea. I’m glad to be able to add an update now. While the AmVeh concept isn’t under active development, it remains on the research agenda at KIER: the AmVeh will go back into development just as soon as they’ve nailed the electrochemical synthesis technology, to supply the car with abundant sustainable ammonia fuel.
You can learn more about the 2017 Power-to-Ammonia conference, as well as older KIER presentations at NH3 Fuel Conferences since 2012 at the NH3 Fuel Association website.
Read the full article at AmmoniaIndustry.com.